High-purity crystalline silicon, high-purity silicon tetrachloride, and processes for producing same
A technology of silicon tetrachloride and manufacturing method, which is applied in the direction of chemical instruments and methods, silicon compounds, silicon halide compounds, etc., can solve the problems of pollution influence, poor thermal expansion coefficient of reactor, damage, etc., and achieve excellent economy and equipment Simple and less waste effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101]
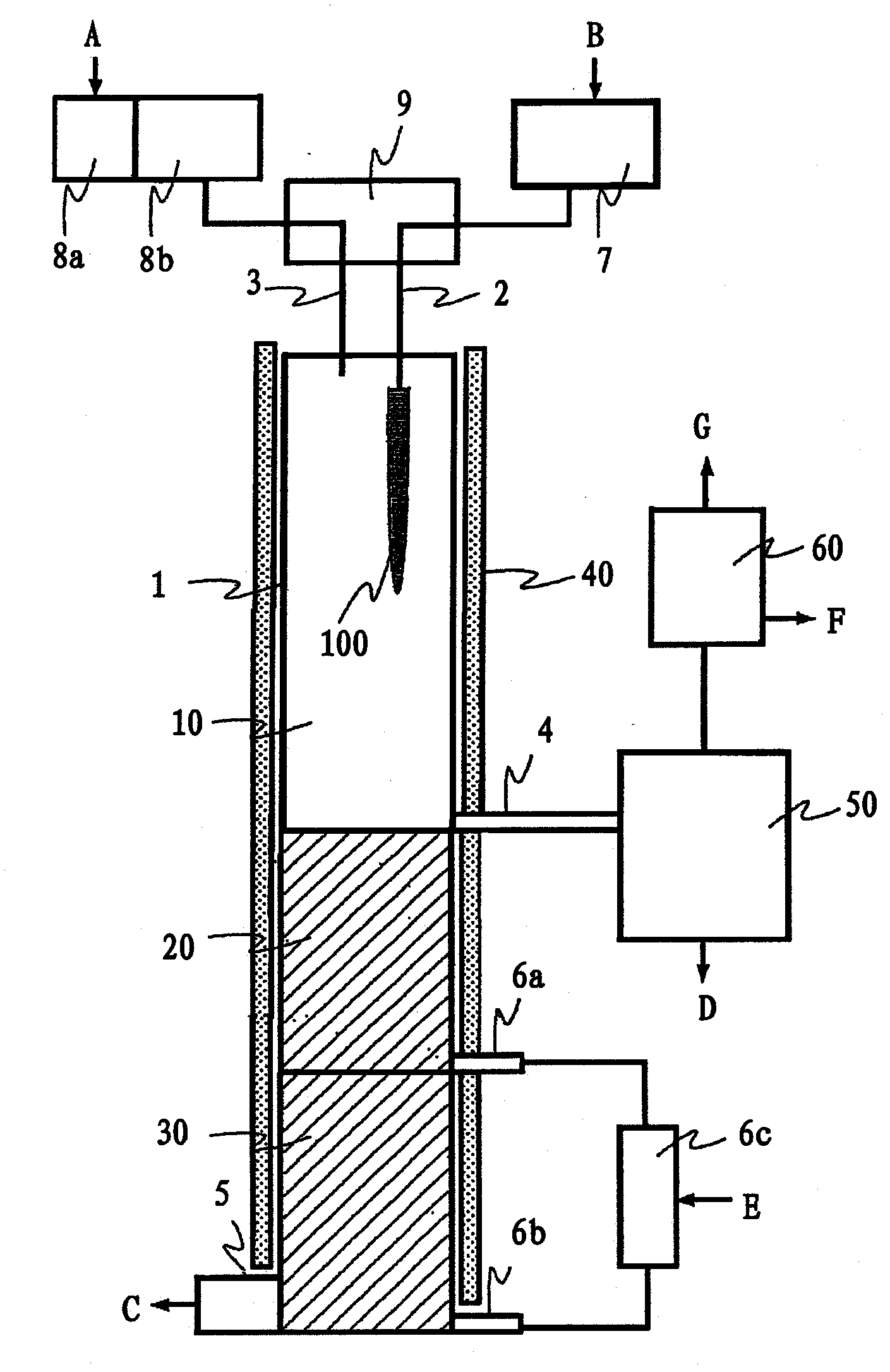
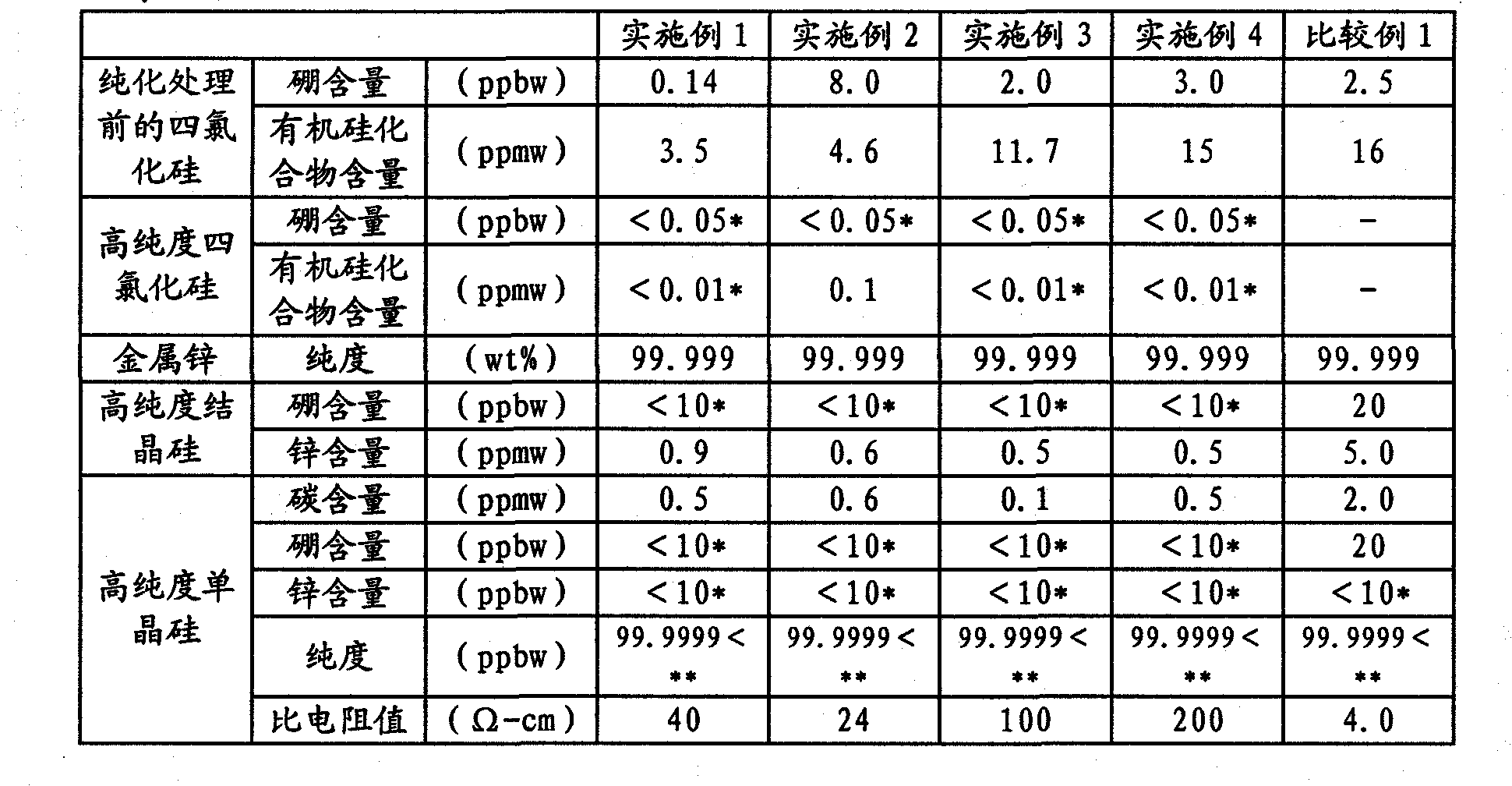
[0102] The silicon tetrachloride used as a raw material is the following industrially used silicon tetrachloride (organosilicon compound content: 3.5ppmw, boron content: 0.14ppbw), which is suitable for industrial use of metal silicon (purity About 98% by weight) reacted with hydrogen chloride and obtained by distillation of the reactant. The above-mentioned silicon tetrachloride was introduced into an adsorption tower filled with 20 kg of silica gel, and circulated at a temperature of 20° C. and a flow rate of 1000 L / h for 24 hours to reduce the boron content. The boron content of silicon tetrachloride after the adsorption treatment is 0.05 ppbw or less which is the detection limit. Also, after vaporizing silicon tetrachloride at 100°C, the silicon tetrachloride was passed through a superheater heated to a temperature of 1100°C with a residence time of 1 second to carbonize and remove the organosilicon compound, and then cooled and collected. Then carry out rectif...
Embodiment 2
[0113]
[0114] The silicon tetrachloride used as raw material is to use the following industrially used silicon tetrachloride (organosilicon compound content: 4.6ppmw, boron content: 8ppbw), which is suitable for industrial metal silicon (purity approx. It is obtained by distillation of the reactant obtained by reacting 98 wt%) with hydrogen chloride. The above-mentioned silicon tetrachloride was introduced into an adsorption tower filled with 20 kg of silica gel, and circulated at a temperature of 20° C. and a flow rate of 1200 L / h for 20 hours to reduce the boron content. The boron content of silicon tetrachloride after the adsorption treatment is 0.05 ppbw or less which is the detection limit. Also, after vaporizing silicon tetrachloride at 100°C, the silicon tetrachloride was passed through a superheater heated to a temperature of 1200°C with a residence time of 1 second to carbonize and remove the organosilicon compound, and then cooled and collected. Then carry out r...
Embodiment 3
[0124]
[0125] The silicon tetrachloride used as a raw material is to use the following industrially used silicon tetrachloride (organosilicon compound content: 11.7ppmw, boron content: 2ppbw), which is suitable for industrial metal silicon (purity approx. It is obtained by distillation of the reactant obtained by reacting 98 wt%) with hydrogen chloride. The above-mentioned silicon tetrachloride was introduced into an adsorption tower filled with 30 kg of silica gel, and circulated at a temperature of 20° C. and a flow rate of 1100 L / h for 24 hours to reduce the boron content. The boron content of the silicon tetrachloride after adsorption treatment is less than or equal to 0.05ppbw of the detection limit. Then, after vaporizing silicon tetrachloride at 100° C., the silicon tetrachloride was passed through a superheated tube heated to a temperature of 1200° C. for a residence time of 3 seconds to carbonize and remove the organosilicon compound, and then cooled and collected...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com