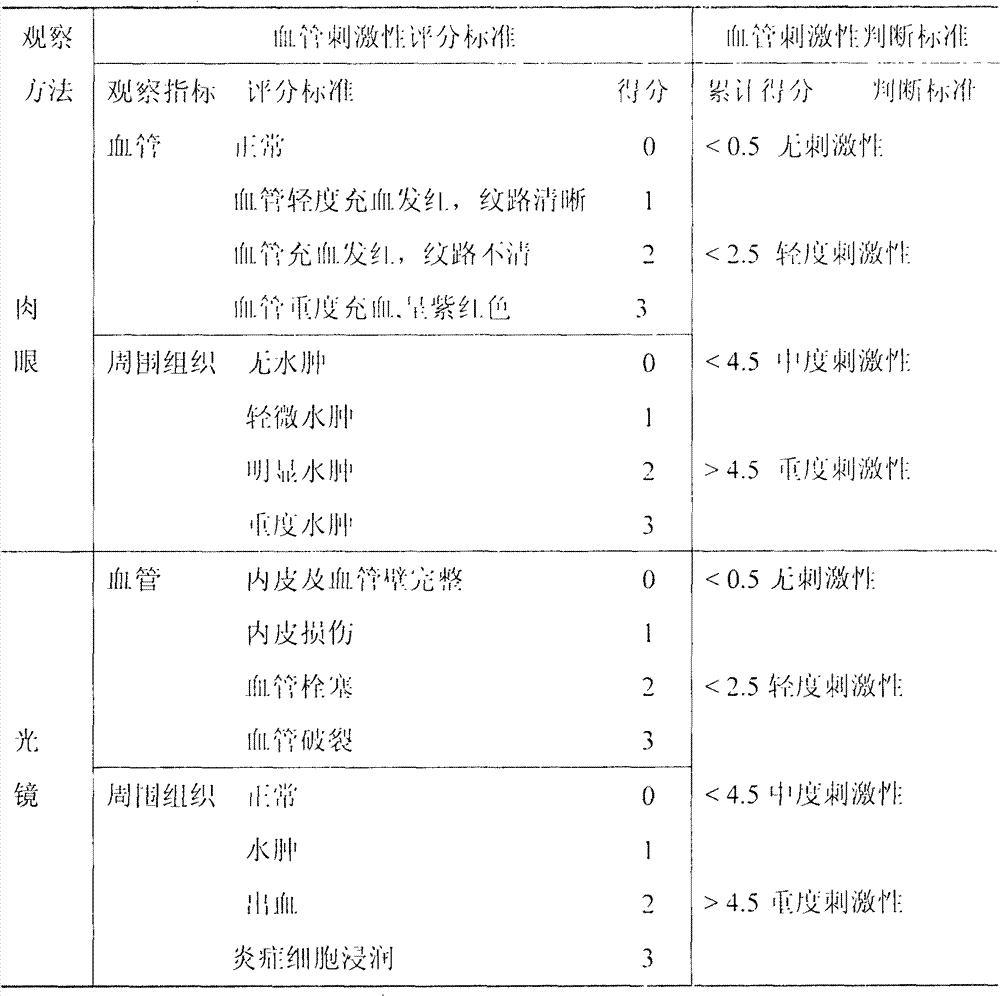
LHRH (luteinizing hormone-releasing hormone antagonist) lyophilized powder injection with improved stability
A freeze-dried powder injection and antagonist technology, applied in the field of LHRH antagonist freeze-dried powder injection, can solve the problems of unfavorable patient acceptance and poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
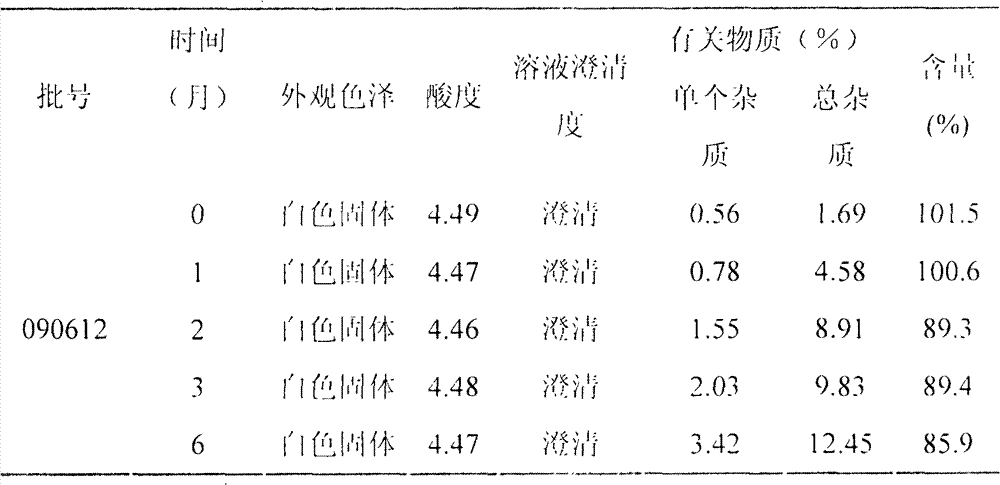
[0023] 0.25mg / ml cetrorelix, 3% w / v mannitol, 0.1% w / v sodium bisulfite, 2% w / v benzyl alcohol, adjust the pH to 4.5 with acetic acid and sodium acetate, and its preparation process is as follows:
[0024] Under sterile conditions, weigh 0.25g of cetrorelix, 30g of mannitol, 1g of sodium bisulfite and 20g of benzyl alcohol, place them in a sterile container, add 80% water for injection, stir to dissolve, and Adjust the pH to 4.5 with sodium acetate, add water for injection to 1000ml, and stir well. Add 25 g of activated carbon for injection and stir for 30 minutes, coarsely filter and decarbonize with a sterile filter, filter with a 0.45 μm microporous membrane, and finally filter with a 0.22 μm microporous membrane. 1ml / bottle), freeze-dried in vacuum, vacuum stoppered, out of the box, and capped. Make each vial equivalent to 0.25 mg cetrorelix.
[0025] 1000 bottles of cetrorelix freeze-dried powder injection (containing cetrorelix 0.25mg) were prepared by the present embo...
Embodiment 2
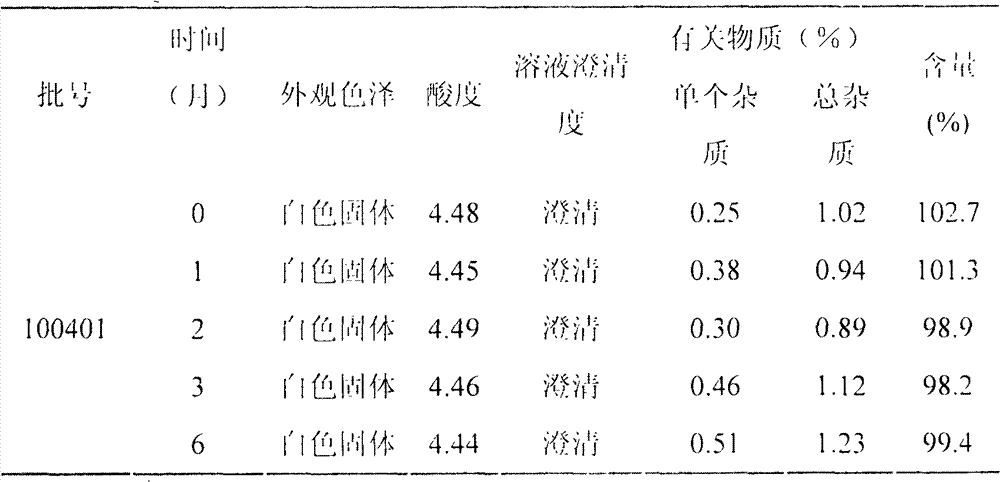
[0057] 0.25mg / ml cetrorelix, 3% w / v mannitol, 0.1% w / v sodium metabisulfite, 0.4% w / v chlorobutanol, adjust the pH to 4.5 with acetic acid and sodium acetate, and the preparation process is as follows :
[0058] Under aseptic conditions, weigh 0.25g of cetrorelix, 30g of mannitol, 1g of sodium metabisulfite and 4g of chlorobutanol, place them in a sterile container, add 80% water for injection, stir to dissolve, and use Adjust the pH to 45 with acetic acid and sodium acetate, add water for injection to 1000ml, and stir well. Add 25 g of activated carbon for injection and stir for 30 minutes, coarsely filter and decarbonize with a sterile filter, filter with a 0.45 μm microporous membrane, and finally filter with a 0.22 μm microporous membrane. 1ml / bottle), freeze-dried in vacuum, vacuum stoppered, out of the box, and capped. Make each vial equivalent to 0.25 mg cetrorelix.
[0059] 1000 bottles of cetrorelix freeze-dried powder injection (containing cetrorelix 0.25mg) were ...
Embodiment 3
[0091] 1mg / ml cetrorelix, 3% w / v lactose, 0.1% w / v sodium metabisulfite, 2% w / v benzyl alcohol, adjust the pH to 4.5 with acetic acid and sodium acetate, and the preparation process is as follows:
[0092] Under sterile conditions, weigh 3 g of cetrorelix, 90 g of lactose, 3 g of sodium metabisulfite and 60 g of benzyl alcohol, place them in a sterilized container, add 80% of the prescription amount of water for injection, stir to dissolve, adjust with acetic acid and sodium acetate When the pH reaches 4.5, add water for injection to 3000ml, and stir well. Add 25g of active carbon for injection and stir for 30min, decarbonize by coarse filtration with a sterilizing filter, filter with a 0.45 μm microporous membrane, and finally filter with a 0.22 μm microporous membrane. 3ml / bottle), freeze-dried in vacuum, vacuum stoppered, out of the box, and capped. Make each vial equivalent to 3 mg cetrorelix.
[0093] Prepare 1000 bottles of cetrorelix freeze-dried powder injection (con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com