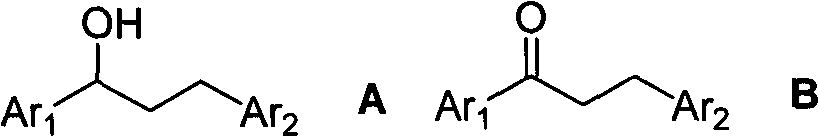Method for synthesizing 1,3-diphenyl-1-propanol compound
A synthesis method and compound technology, applied in the field of cross-coupling reaction between secondary alcohol and primary alcohol, can solve the problems of unsuitable ligands, need special preparation, waste of raw materials, etc., so as to reduce the difficulty of purification and post-processing, and reduce the impact of the environment. , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Under nitrogen, ferrocene 0.1mmol, 18.6mg, Cs 2 CO 3 1.0mmol, 32.6mg, 1-phenyl-ethanol 10.0mmol, 1.22g and benzyl alcohol 15.0mmol, 1.62g were sequentially added to 6.7mL of anhydrous m-xylene; stirred at 125°C for 8h. Cool to NH 4 Neutralize the reaction solution with a saturated Cl solution, extract it with ethyl acetate, and concentrate the extract in vacuo until there is no ethyl acetate odor; purify it by 100-200 mesh forward silica gel column chromatography, n-hexane: ethyl acetate = 40:1 After elution, the product 1,3-diphenyl-1-propanol 1.55g, 7.3mmol, yield: 73% can be obtained.
Embodiment 2
[0039] Under nitrogen, ferrocene formaldehyde 0.5mmol, 107.0mg, NaOH 2.0mmol, 80.0mg, 1-phenyl-ethanol 10.0mmol, 1.22g and 4-methoxybenzyl alcohol 10.0mmol, 1.38g were added in sequence for 10.0 mL of anhydrous p-xylene; at 130 ° C, stirred for 24h. Cool to NH 4 The reaction solution was neutralized with a saturated Cl solution, extracted with ethyl acetate, and the extract was concentrated in vacuo until there was no ethyl acetate smell. Purified by 100-200 mesh forward silica gel column chromatography, eluting with n-hexane:ethyl acetate=30:1, the product 1-phenyl-3-(4'-methoxyphenyl)-1-propane can be obtained Alcohol 2.33g, 9.6mmol, yield: 96%.
Embodiment 3
[0041] Under nitrogen, ferrocenemethanol 1.0mmol, 216.1mg, KOH 1.5mmol, 84.2mg, 1-(4'-methylphenyl)-ethanol 10.0mmol, 1.36g and 4-chlorobenzyl alcohol 20.0mmol, 2.85 g, successively added to 5.0 mL of anhydrous o-xylene. At 135°C, stir for 12h; cool, add NH 4 Neutralize the reaction solution with a saturated Cl solution, extract it with ethyl acetate, and concentrate the extract in vacuo until there is no ethyl acetate odor; purify it by 100-200 mesh forward silica gel column chromatography, n-hexane: ethyl acetate = 38:1 After elution, the product 1-(4'-methylphenyl)-3-(4'-chlorophenyl)-1-propanol 2.11g, 8.1mmol, yield: 81% can be obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com