Anti-EGFR (epidemic growth factor receptor) humanized antibody L2-H3 and coding gene and application thereof
A technology encoding genes and antibodies, applied in applications, antibodies, gene therapy, etc., can solve the problems of murine glycosylation pattern, prolong the half-life of antibodies, and have many hypersensitivity reactions, achieve broad application prospects, and ensure the effect of anti-tumor effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1. Acquisition of genes encoding light chain and heavy chain variable regions of antibodies
[0049] According to computer simulation, using the amino acid sequence of the mouse-human chimeric antibody cetuximab as a template, the mouse FR surface gene was humanized to design and synthesize the amino acid sequence of the light chain and the "variable region + heavy chain" of the heavy chain Amino acid sequence of constant region 1".
[0050] The antibody of the present invention consists of light chain L2 and heavy chain H3; heavy chain H3 consists of heavy chain variable region (VH), heavy chain constant region 1 (CH1), hinge region, heavy chain constant region 2 (CH2) and heavy chain constant Region 3 (CH3) consists of (H3=VH+CH1+hinge+CH2+CH3). The antibody of the present invention is referred to as L2-H3.
[0051] Light chain L2: the amino acid sequence is shown in SEQ ID NO: 1; the coding gene sequence is shown in positions 85-726 of SEQ ID NO: 2;
[0052]...
Embodiment 2
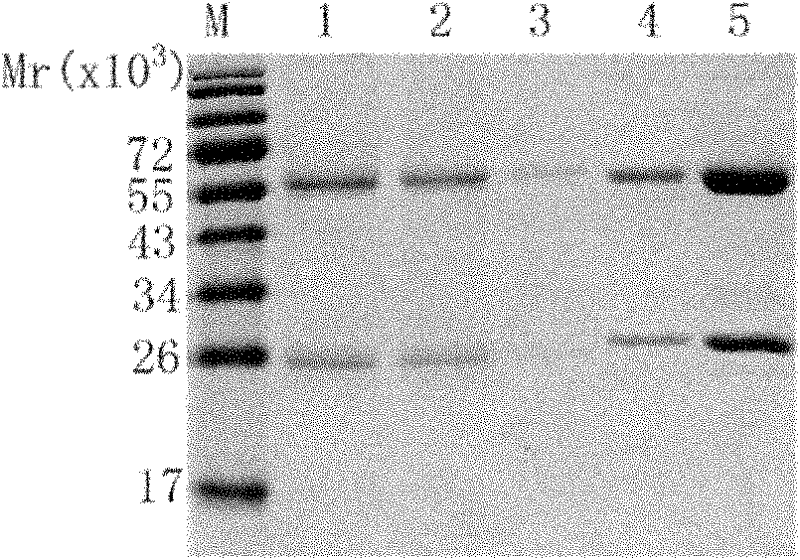
[0073] Example 2, Expression and Purification of Antibodies
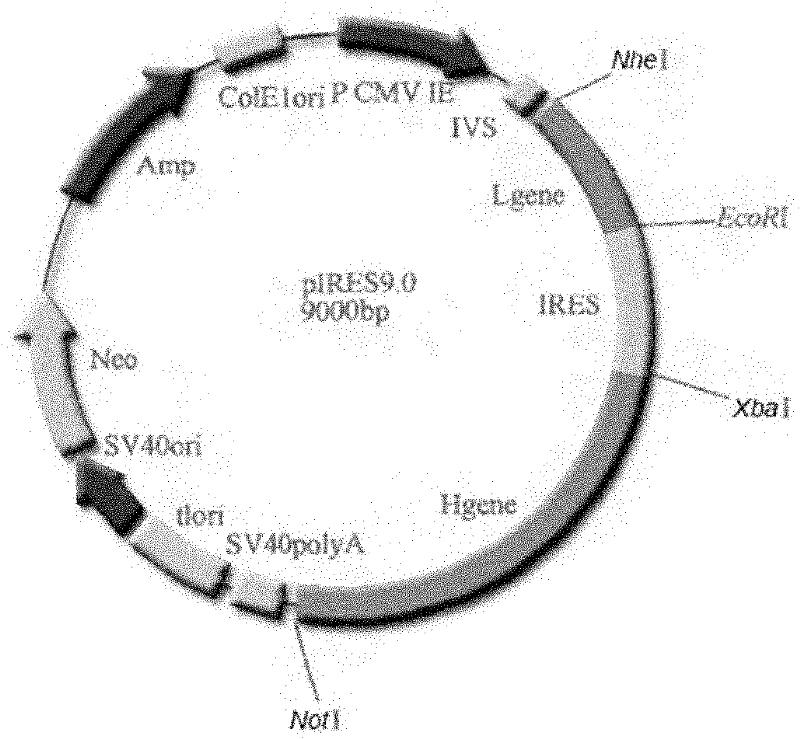
[0074] 1. Construction of recombinant expression vectors:
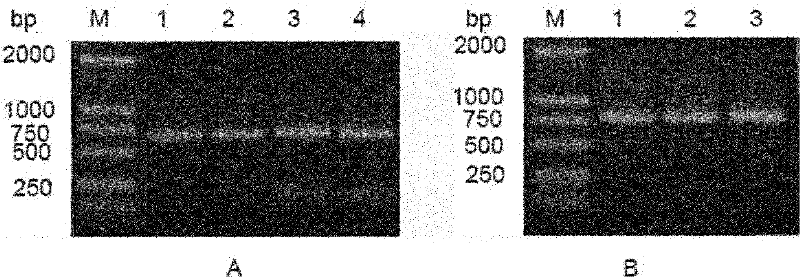
[0075] Recombinant vector pMD18-T / L2 and pIRES double expression vector were digested with corresponding restriction enzymes (Nhe I and EcoR I) respectively, and after agarose gel electrophoresis, the target fragment was recovered and purified; the light chain gene fragment L2 Mix well with the carrier fragment, and react at 16°C for 12h under the action of the ligation reagent. Transform Escherichia coli DH5a, pick a single clone, extract the plasmid, and sequence and identify it. The result: between the Nhe I and EcoR I restriction sites of the vector pIRES (along the direction from the Nhe I restriction site to the EcoR I restriction site ) was inserted into the light chain coding gene shown in nucleotides 9-729 of SEQ ID NO: 2, indicating that the constructed recombinant vector was correct, which was designated as the recombinant expression vector pIRE...
Embodiment 3
[0113] Embodiment 3, the function of antibody
[0114] 1. Biacore detects the binding ability of antibody and antigen
[0115] Sensor Chip CM5 was purchased from BD Company, the product catalog number is Br-1000-14; BD BioCoat TM Matrigel TM Invasion Chamber is purchased from BD Company, and the product catalog number is 354480.
[0116] EGFR protein was purchased from Sigma Company, catalog number E2645-500UN.
[0117] The affinity of the antibody to EGFR was determined with Biacore3000 equipment. Prepare 10mmol / L NaAc diluted EGFR protein with different pH values (4.0, 4.5, 5.0 and 5.5), pre-concentrate on the CM5 chip, and select the NaAc diluted protein with the optimal pH value. The purified antibody (i.e. the eluate obtained in step 2 in Example 2) was covalently coupled to the CM5 sensor chip, the mobile phase was HBS-EP (pH7.4), the flow rate was 20 μl / min, and five concentrations were taken Detection of binding affinity of antibodies (0, 10.55, 21.1, 42.2 and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com