Method for producing germanium tetrafluoride
A technology of germanium tetrafluoride and its manufacturing method, which is applied to the preparation of fluorides, germanium halides, etc., and can solve problems such as the control of difficult reactions and the improvement of manufacturing efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
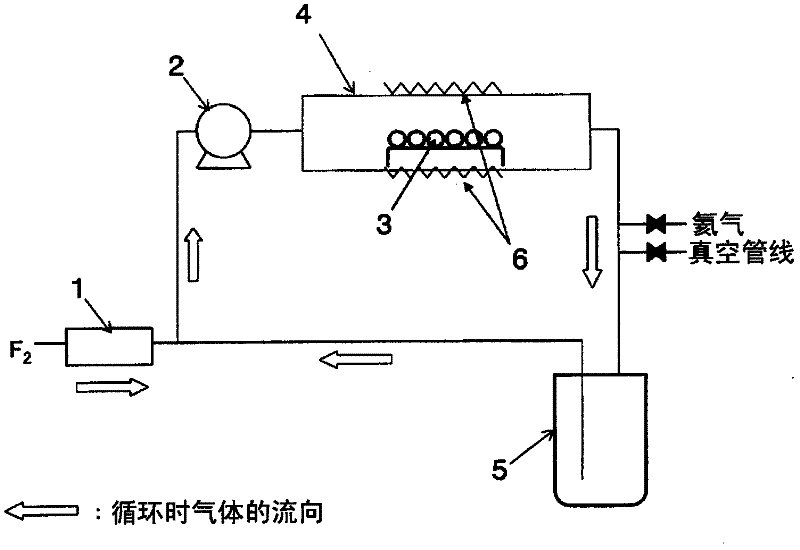
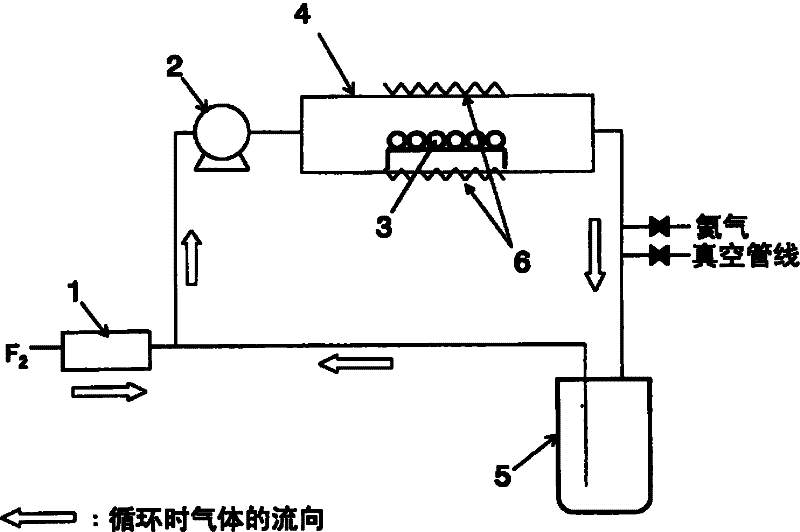
[0035] 1000 g of metal germanium 3 powder with a purity of 99.99% was filled in the center of a tubular reactor 4 made of nickel with an inner diameter of 80 mm and a length of 1000 mm. The inside of the system was replaced with a vacuum, and the temperature of the outer wall of the reactor 4 was set to 200° C., and helium gas was introduced into the system to make it 80 kPa. The cooling trap 5 was cooled to -60°C. Next, set the circulating flow rate of pump 2 to 6L / min, and pass F 2 Fluorine gas was supplied by a mass flow controller 1 at a flow rate of 400 cc / min, and the reaction was performed for 10 hours. Thereafter, the generated gas trapped in the cooling trap 5 was analyzed by FT-IR (IG-1000 manufactured by Otsuka Electronics Co., Ltd.) and ultraviolet spectrophotometer (U-2810 manufactured by Hitachi), and it was confirmed that germanium tetrafluoride generate. In addition, the concentration of fluorine gas in the outlet gas of reactor 4 was analyzed using an ultra...
Embodiment 2
[0037] 500 g of metal germanium 3 powder with a purity of 99.99% was filled in the center of a tubular reactor 4 made of nickel with an inner diameter of 80 mm and a length of 1000 mm. Vacuum replacement was performed in the system, and the temperature of the outer wall of the reactor 4 was set at 150° C., and helium gas was introduced into the system to make it 120 kPa. The cooling trap 5 was cooled to -60°C. Next, set the circulating flow rate of pump 2 to 10L / min, and pass F 2 Fluorine gas was supplied at a flow rate of 300 cc / min by a mass flow controller 1, and the reaction was performed for 10 hours. Thereafter, the generated gas trapped in the cooling trap 5 was analyzed by FT-IR (IG-1000 manufactured by Otsuka Electronics Co., Ltd.) and ultraviolet spectrophotometer (U-2810 manufactured by Hitachi), and it was confirmed that germanium tetrafluoride generate. In addition, the concentration of fluorine gas in the outlet gas of reactor 4 was analyzed using an ultraviol...
Embodiment 3
[0039] 2000 g of metal germanium 3 powder with a purity of 99.99% was filled in the center of a reactor 4 made of nickel with an inner diameter of 130 mm and a length of 700 mm. The inside of the system was replaced with a vacuum, and the temperature of the outer wall of the reactor 4 was set at 250° C., and helium gas was introduced into the system to make it 101 kPa. The cooling trap 5 was cooled to -60°C. Next, set the circulating flow rate of pump 2 to 15L / min, and pass F 2Fluorine gas was supplied at a flow rate of 700 cc / min by a mass flow controller 1, and the reaction was performed for 10 hours. Thereafter, the generated gas trapped in the cooling trap 5 was analyzed by FT-IR (IG-1000 manufactured by Otsuka Electronics Co., Ltd.) and ultraviolet spectrophotometer (U-2810 manufactured by Hitachi), and it was confirmed that germanium tetrafluoride generate. In addition, the concentration of fluorine gas in the outlet gas of reactor 4 was analyzed using an ultraviolet ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com