Hydroxybutyrate ester and medical use thereof
A technology of hydroxybutyrate and hydroxybutyl, applied in the field of hydroxybutyrate, can solve problems such as impracticality of direct administration, acidosis and danger
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The synthesis of embodiment 1 (3R)-hydroxybutyl (3R)-hydroxybutyrate
[0062]
[0063] Ethyl(3R)-hydroxybutyrate (approximately 3kg), (R)-1,3-butanediol (approximately 1.5kg) and solid-supported Candida antarctica lipase B (approximately 300g) were dissolved in a 20L Mix in a rotary evaporator flask, then place in a large on the evaporator. The system was rotary evacuated to 8-10 Torr at 40-45 °C until the diol was depleted (e.g. by 1 H NMR spectroscopic analysis; about 3 days). The crude material was filtered (clean), the enzyme was isolated, and excess ethyl (3R)-hydroxybutyrate was removed by evaporation (2-3 Torr and final pressure and temperature of 80-85°C). Cooling water was circulated throughout [-5°C during reaction, +5°C during ethyl(3R)-hydroxybutyrate removal]. Activated charcoal (quantity of 8 spatulas) was added, the mixing on the rotary evaporator was continued for 15 minutes, and then the pure mixture was passed through Filter and pour the pr...
Embodiment 2
[0064] Example 2 : (3R)-Hydroxybutyl(3R)-hydroxybutyrate - in vivo testing of a calorie-controlled diet
[0065] Young adult male Wistar rats (starting body weight 70 g) (Harlan UK Limited) (n=50) were housed at approximately 20°C under a 12h:12h light:dark cycle. Feed with standard laboratory chow (Chow) (SDS, Essex, UK) before starting the experimental diet: (a) normal "Western" diet (Western) (n=20) with 34% caloric From added palmitate, (b) high carbohydrate (CHO) (n=10) with 70% of calories from added corn starch or (c) (3R)-hydroxybutyl(3R)-hydroxybutyric acid Ester diet (monoester) (n=20) with 30% of calories from (3R)-hydroxybutyl (3R)-hydroxybutyrate.
[0066] The macronutrient indicators for these three meals are shown below. All meals contained the same energy in kCal / g but had different macronutrients.
[0067] Table 1
[0068]
[0069] Meals and monoesters were prepared at Oxford University. Water was quoted ad libitum. The research project was approv...
Embodiment 3
[0076] Example 3 : (3R)-Hydroxybutyl (3R)-hydroxybutyrate-in vivo assay of a meal-feeding diet test
[0077] Example 2 was repeated using the same diet as shown in Table 1, except that the rats were meal-fed. Therefore, the rats in this example can freely choose how much food to eat at each meal, instead of controlling calories as in Example 2.
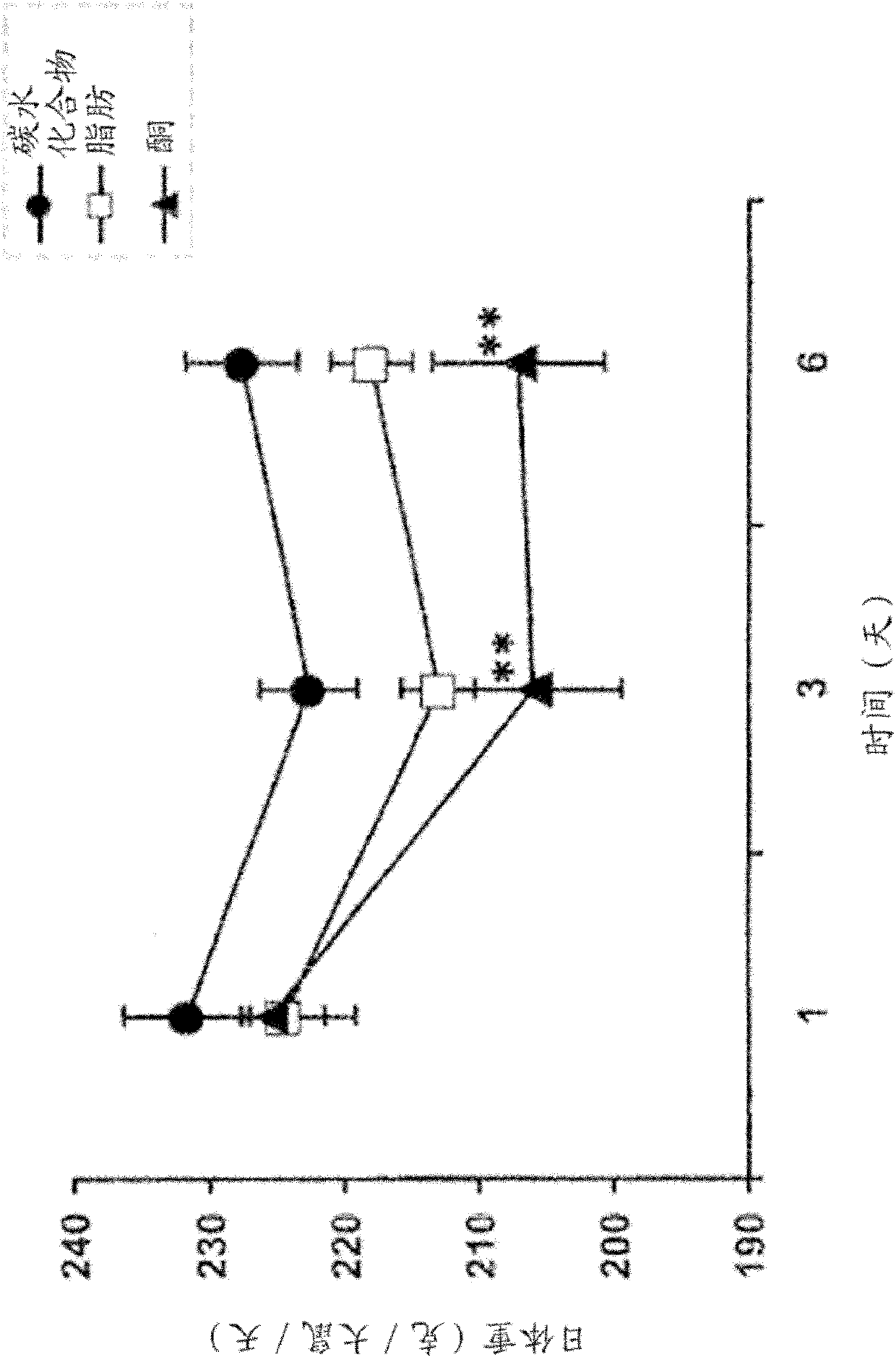
[0078] Daily body weight (in g per rat per day) was plotted over time for each rat in the three dietary groups during the first 6 days of testing. The resulting graph is shown in figure 1 middle. One-way analysis of variance using Tukey-Kramer multiple comparison test (n=8 per group, ** p<0.001). In rats fed the monoester diet, a significant reduction in body weight was observed from day 3 to day 6. Rats fed the carbohydrate diet maintained high body weight throughout the feeding period.
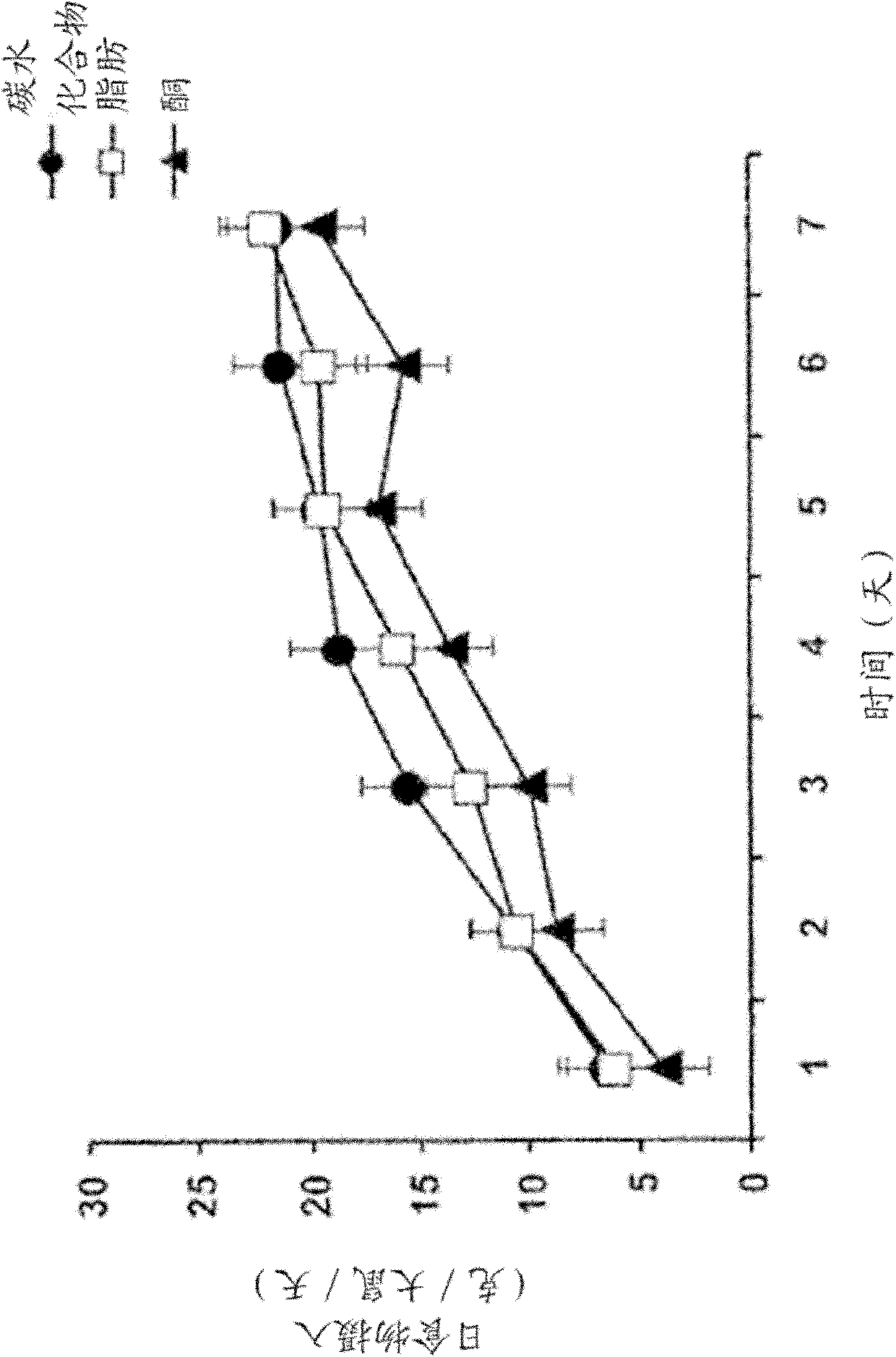
[0079] Rats fed the monoester diet ate less and lost more body weight than rats on the other two diets. Daily food intake (in g per ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com