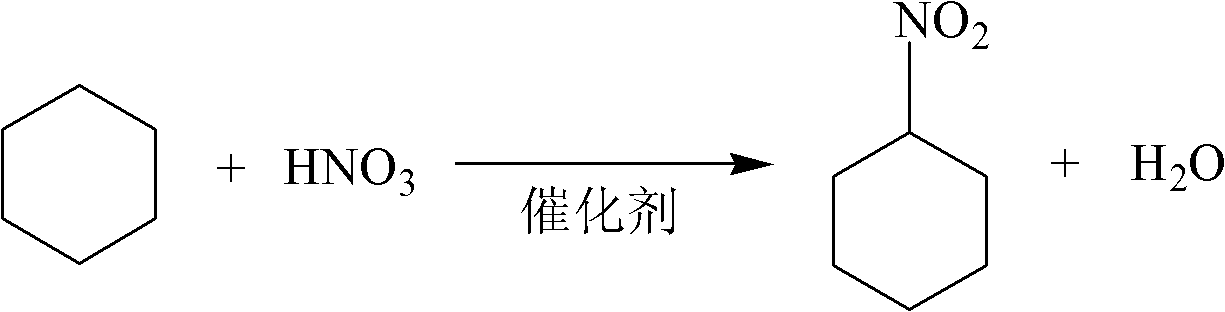
Nitration catalyst and preparation method and application thereof
A catalyst, liquid-phase nitration technology, applied in the preparation of nitro compounds, catalysts for physical/chemical processes, chemical instruments and methods, etc., can solve the problems of high reaction cost, expensive fluorophosphate, catalyst deactivation, etc., and achieve simplification Process, improve resource utilization, simple effect of separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 16 g of ZrOCl 2 ·8H 2 O and 20g Al(NO 3 ) 3 ·9H 2 O was dissolved in distilled water to prepare a solution with a concentration of 1mol / L, and then a certain amount of 6mol / L ammonia water was added under the condition of rapid stirring to control the pH value of the solution to 9.0 to obtain Zr(OH) 4 Precipitation and Al(OH) 3 , the precipitate was aged at 30 °C for 24 hours, filtered, and then washed repeatedly with deionized water to remove chloride ions until the AgNO 3 Solution test filtrate free of chloride ions. The resulting precipitate was dried at 110°C for 12 hours to obtain a solid powder, which was ground and impregnated with 200ml of 1.0mol / L sulfuric acid solution for 24 hours, then filtered off excess sulfuric acid, dried at 110°C and placed in a muffle furnace The temperature was raised to 550° C. at a heating rate of 5° C. per minute, and the calcination was continued at this temperature for 3 hours to obtain the catalyst for catalyzing the liqui...
Embodiment 2
[0033] 16 g of ZrOCl 2 ·8H 2 O and 5 g Al(NO 3 ) 3 ·9H 2 O was dissolved in distilled water to prepare a solution with a concentration of 1mol / L, and then a certain amount of 6mol / L ammonia water was added under the condition of rapid stirring to control the pH value of the solution to 9.0 to obtain Zr(OH) 4 Precipitation and Al(OH) 3 , the precipitate was aged at 10°C for 24 hours, then filtered, and then washed repeatedly with deionized water to remove chloride ions until the AgNO 3Solution test filtrate free of chloride ions. The obtained precipitate was dried at 110°C for 12 hours to obtain a solid powder, which was ground and impregnated with 150ml of 1.0mol / L sulfuric acid solution for 24 hours, then filtered off excess sulfuric acid, dried at 110°C and placed in a muffle In the furnace, the temperature was raised to 650° C. at a rate of 5° C. per minute, and the calcination was continued at this temperature for 3 hours to obtain the catalyst for catalyzing the liq...
Embodiment 3
[0035] 16 g of ZrOCl 2 ·8H 2 O and 5 g Al(NO 3 ) 3 9H 2 O was dissolved in distilled water to prepare a solution with a concentration of 1mol / L, and then a certain amount of 6mol / L ammonia water was added under the condition of rapid stirring to control the pH value of the solution to 9.0 to obtain Zr(OH) 4 Precipitation and Al(OH) 3 , the precipitate was aged at 10°C for 24 hours, then filtered, and then washed repeatedly with deionized water to remove chloride ions until the AgNO 3 Solution test filtrate free of chloride ions. The obtained precipitate was dried at 110°C for 12 hours to obtain a solid powder, which was ground and impregnated with 150ml of 1.0mol / L sulfuric acid solution for 24 hours, then filtered off excess sulfuric acid, dried at 110°C and placed in a muffle In the furnace, the temperature was raised to 650° C. at a rate of 5° C. per minute, and the calcination was continued at this temperature for 3 hours to obtain the catalyst for catalyzing the liq...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com