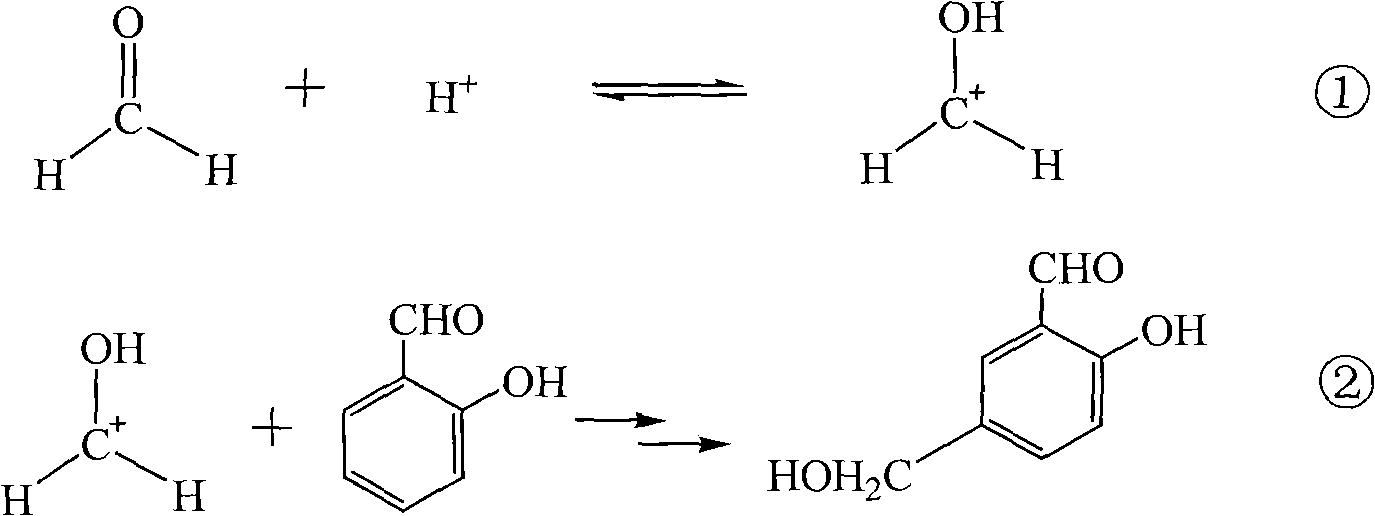
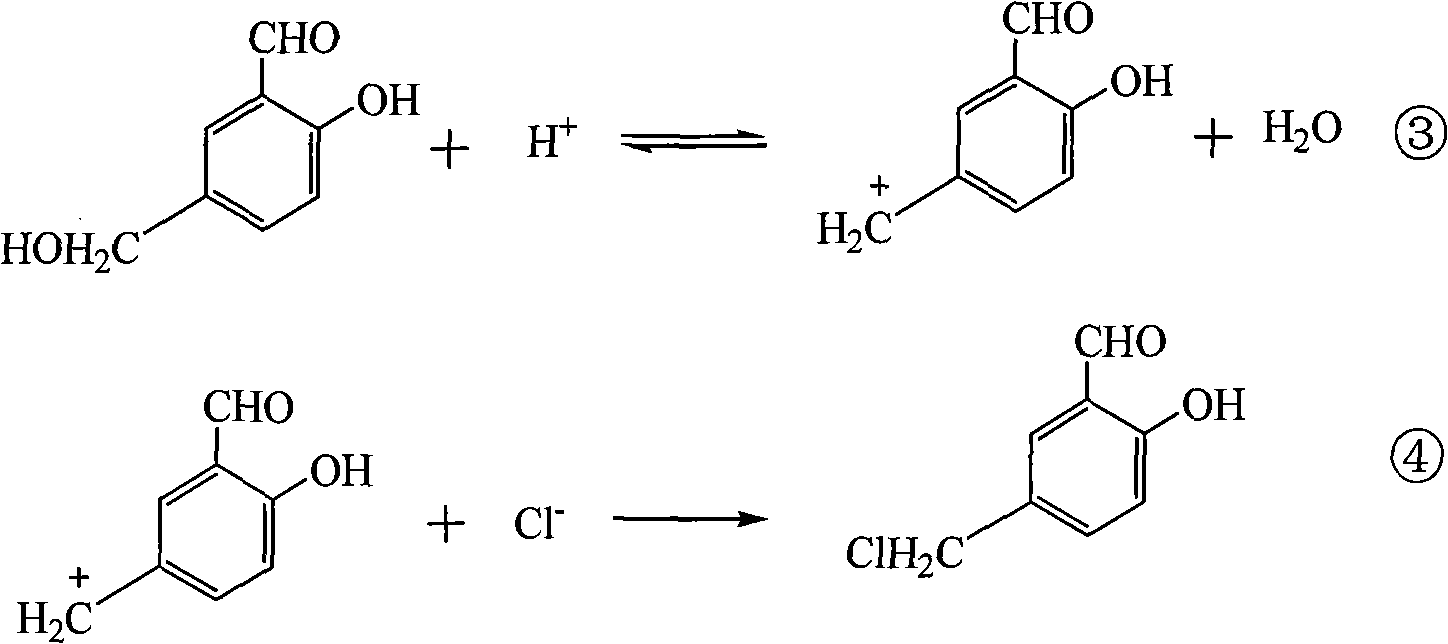
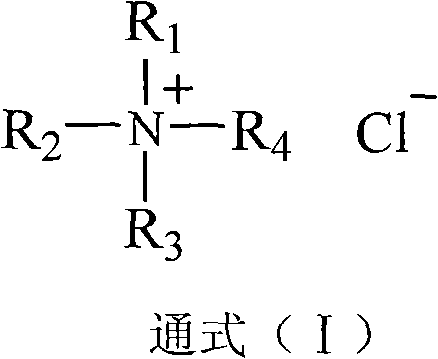
Preparation method of 5-chloromethyl salicylaldehyde
A technology of chloromethyl salicylaldehyde and salicylaldehyde, which is applied in the field of preparation of 5-chloromethyl salicylaldehyde, can solve the problems of difficult drying, low yield of 5-chloromethyl salicylaldehyde, and difficult Washing and other issues, to achieve the effect of improving the conversion rate of raw materials, increasing the probability of participating in chloromethylation reactions, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In a 500mL reaction flask, put 50g of salicylaldehyde, 55g of formaldehyde solution with a concentration of 37% by mass and 2.5g of triethylbenzyl ammonium chloride, turn on the mixer, control the temperature at 5-15°C, and then add Concentration is 37~38% hydrochloric acid 200mL, after stirring reaction for 12 hours, feed 37g of hydrogen chloride gas into reaction system continuously within 10 hours. Thereafter, the airtight reaction was continued for 24 hours until the end. The crude product of 5-chloromethyl salicylaldehyde was obtained by discharging and filtering, and was put into a washing flask, and 150 g of water was added, and the temperature of the material in the washing flask was controlled at 40-45° C., and stirred and washed for 1 hour. Afterwards, repeat washing with water once; then wash once with aqueous sodium bicarbonate solution with a mass percentage concentration of 8-10% at room temperature, and finally wash once more with water, and vacuum-dry th...
Embodiment 2
[0023] According to the operating method and steps of Example 1, 55 g of formaldehyde solution with a mass percent concentration of 37% was replaced with 23 g of paraformaldehyde, and the reaction time was extended to 96 hours to obtain 67.1 g of 5-chloromethyl salicylaldehyde product, The melting point is 82-85°C, and the yield is 96%.
Embodiment 3
[0025] According to the operation method and steps of Example 1, 55 g of formaldehyde solution and 1 g of triethylbenzyl ammonium chloride with a mass percent concentration of 37% were replaced with 23 g of paraformaldehyde and 3.8 g of tri-n-butyl benzyl ammonium chloride 67.2 g of 5-chloromethyl salicylaldehyde was obtained, with a melting point of 82-86° C. and a yield of 96.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com