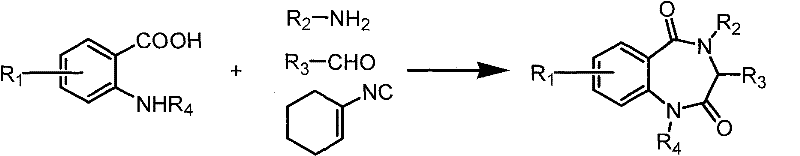
Preparation method of benzodiazepine compound
A technology of benzodiazepines and compounds, which is applied in the field of preparation of benzodiazepine compounds, can solve the problems of cumbersome reaction steps and destruction of isonitrile components, etc., and achieve the effects of shortening reaction steps, low synthesis cost, and easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
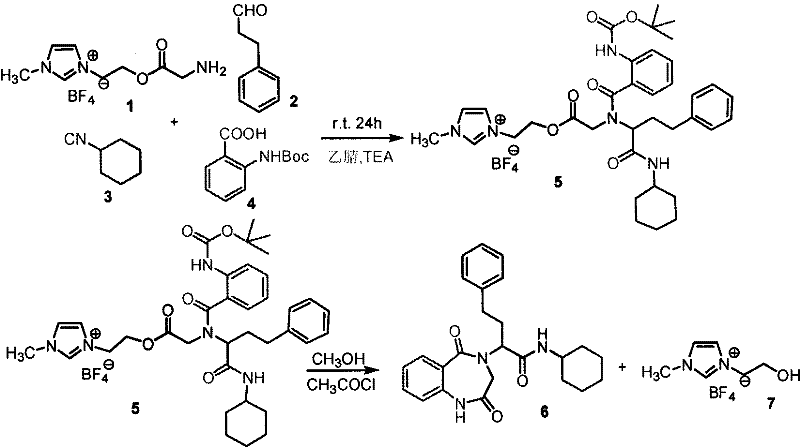
[0019] Embodiment 1, the synthesis of N-cyclohexyl-2-(2,5-dioxo-1,4-benzodiazepine)-4-phenylbutanamide
[0020] 0.0286g (0.100mmol) amino functionalized ionic liquid boron tetrafluoride 1-[2-(aminoacetoxy) ethyl]-3-methylimidazolium salt, 0.0160g (0.120mmol) phenylpropanal was added to 50ml In the round bottom flask, pass nitrogen protection, add 10.0ml of anhydrous acetonitrile, add 2.00ml of triethylamine dropwise, stir at room temperature for 30min, cool to 0°C, add 0.0237g (0.100mmol) Boc-anthranilic acid in turn , 0.0109 g (0.100 mmol) cyclohexylisonitrile. After stirring at room temperature for 24 h, the filtrate was evaporated to dryness to obtain the Ugi product supported by ionic liquid, which was washed 3 times with 10.0 ml of anhydrous ether and 3 times with 10.0 ml of toluene.
[0021] After adding 5.00 ml of anhydrous methanol and 0.0142 ml (0.200 mmol) of acetyl chloride into the reaction bottle and stirring at room temperature for 24 h, a benzodiazepine compoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com