Micelles and freeze-dried preparations of diblock polymer-supported taxane medicines and preparation and use thereof
A technology of diblock polymers and taxanes, which can be applied in the directions of drug combination, drug delivery, medical preparations of inactive ingredients, etc., can solve the problem of death, increase in toxic and side effects, and limit taxane drugs such as paclitaxel Clinical application and other problems, to achieve the effect of simple preparation process, easy operation of preparation process and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] (1) Combine 92mg of methoxy polyethylene glycol poly(ε-caprolactone) copolymer (MPEG 2000 -PCL 2000 ) And 8mg paclitaxel dissolved in 2ml absolute ethanol, heated or ultrasonically stirred until completely dissolved, to obtain a clear solution A;
[0075] (2) Rotate and evaporate the clarified solution A under heating to remove the organic solvent to make it a transparent gel B;
[0076] (3) Add 5ml of preheated water for injection to gel B, make it clear and transparent under heating and stirring, and pass through a 0.22μm filter membrane to prepare micelle C with a particle size of 10nm-100nm;
[0077] (4) The micelle C is lyophilized to obtain the final stable nano-medicine lyophilized powder D.
[0078] Before the lyophilized powder is used clinically, the nano-sized micelles formed automatically under the condition of heating with water for injection.
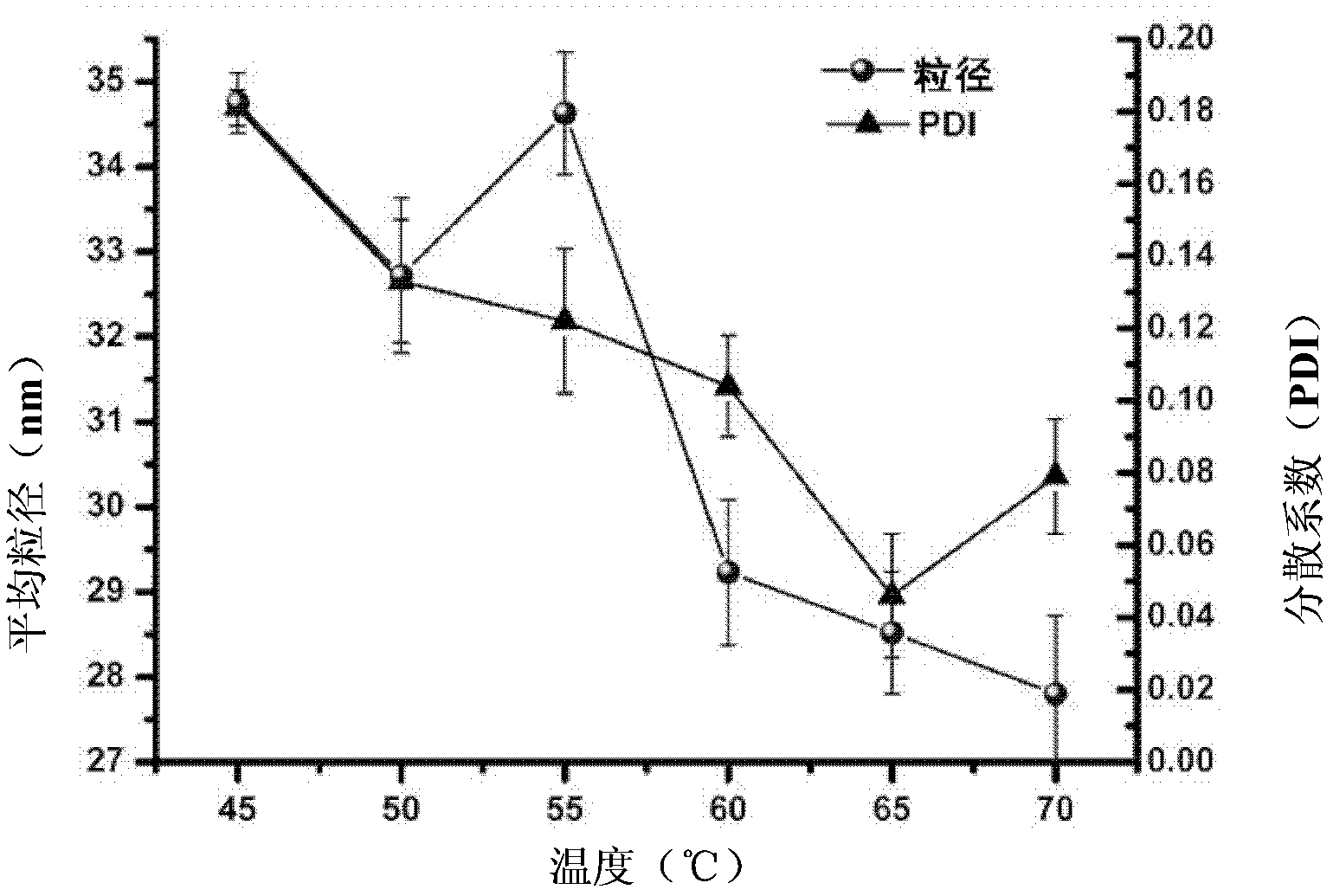
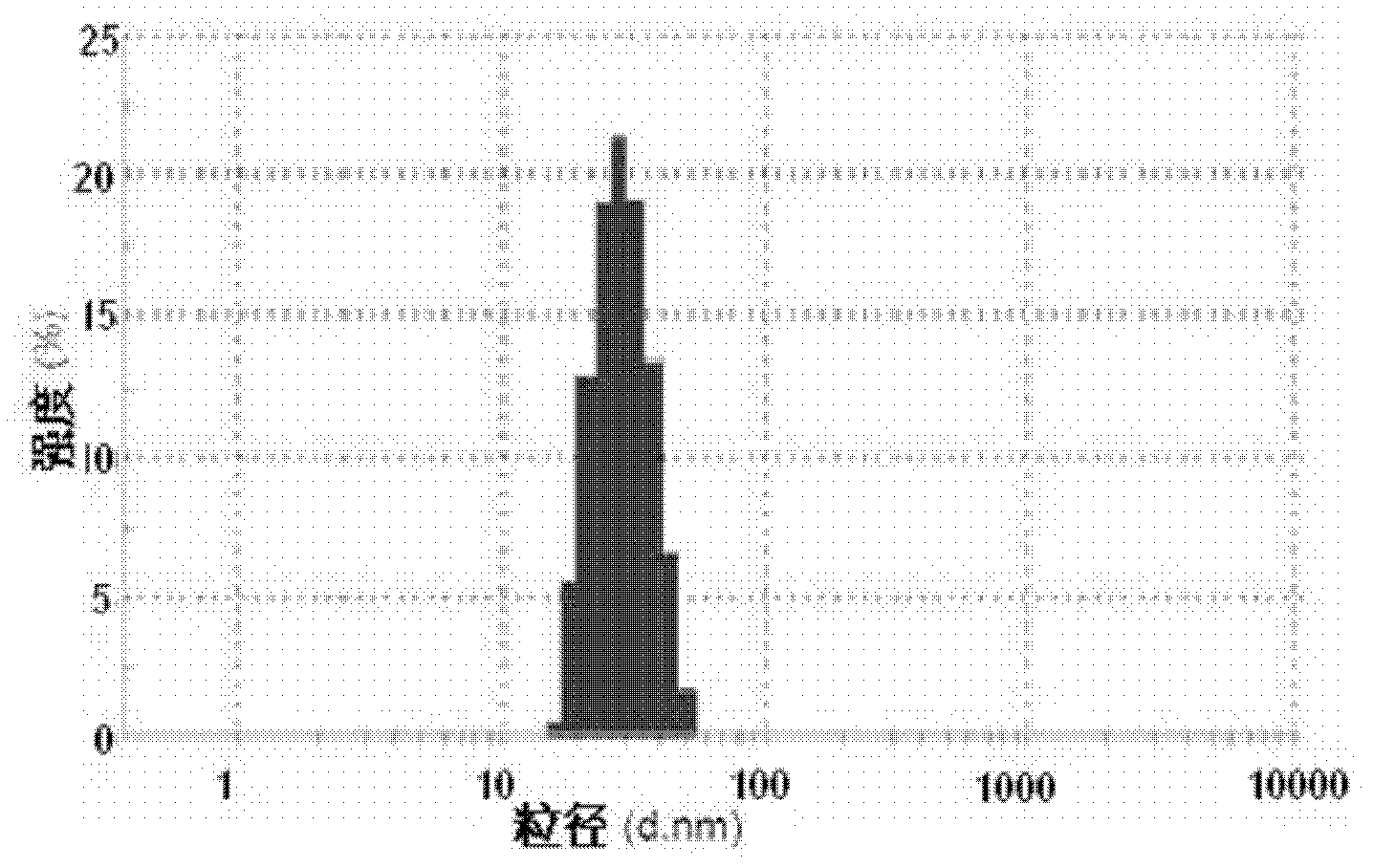
[0079] The resulting micelle C, lyophilized powder D and the final reconstituted micelle, see figure 2 . The average diam...
Embodiment 2
[0081] (1) Put 85mg of methoxy polyethylene glycol poly(ε-caprolactone) copolymer (MPEG 2000 -PCL 2000 ) And 15mg of docetaxel are dissolved in 2ml of absolute ethanol, heated and stirred until completely dissolved to obtain a clear solution A;
[0082] (2) Rotate and evaporate the clarified solution A under heating to remove the organic solvent to make it a transparent gel B;
[0083] (3) Add 5ml of preheated water for injection to gel B, make it clear and transparent under heating and stirring, and pass through a 0.22μm filter membrane to prepare micelle C with a particle size of 10nm-100nm;
[0084] (4) The micelle C is lyophilized to obtain the final stable nano-medicine lyophilized powder D.
[0085] Before the lyophilized powder is used clinically, the nano-sized micelles formed automatically under the condition of heating with water for injection.
[0086] The properties of the obtained micelle C, lyophilized powder D and the final reconstituted micelle are the same figure 2 the...
Embodiment 3
[0088] (1) Combine 92mg of methoxy polyethylene glycol poly(ε-caprolactone) copolymer (MPEG 2000 -PCL 2000 ) And 5mg paclitaxel are dissolved in 2ml absolute ethanol, heated and stirred until completely dissolved to obtain a clear solution A;
[0089] (2) Rotate and evaporate the clarified solution A under heating to remove the organic solvent to make it a transparent gel B;
[0090] (3) Add 5ml of preheated water for injection to gel B, make it clear and transparent under heating and stirring, and pass through a 0.22μm filter membrane to prepare micelle C with a particle size of 10nm-100nm;
[0091] (4) The micelle C is lyophilized to obtain the final stable nano-medicine lyophilized powder D.
[0092] Before the lyophilized powder is used clinically, the nano-sized micelles formed automatically under the condition of heating with water for injection. The resulting micelles have a drug loading capacity of 5.09% and an encapsulation efficiency of 99.17%; the resulting micelles C, lyop...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com