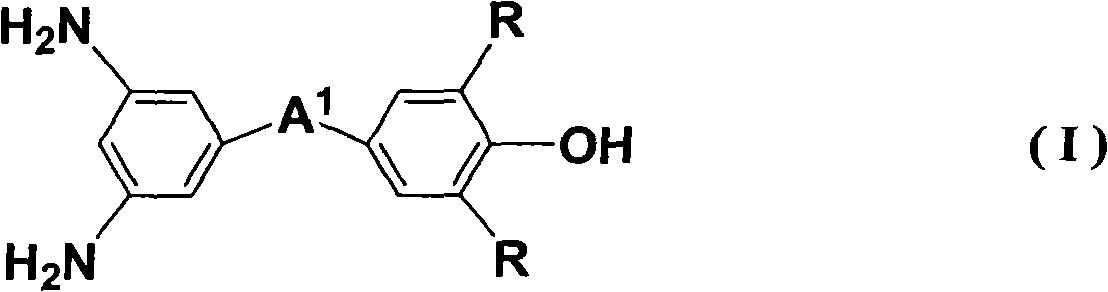
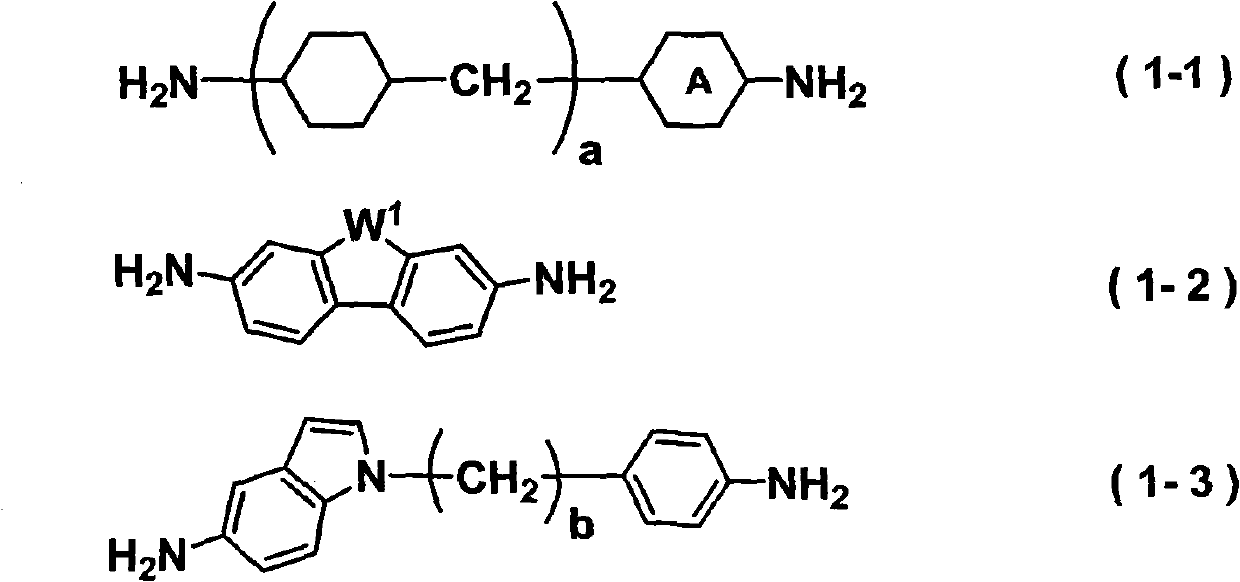
Liquid crystal aligning agent, liquid crystal alignment layer and liquid crystal display device
A technology of liquid crystal alignment agent and carbon number, applied in liquid crystal materials, instruments, organic chemistry, etc., can solve the problems of brightness or contrast reduction, halftone brightness inversion, narrow viewing angle, etc., and achieve residual DC suppression and high voltage retention rate , Improve the effect of voltage retention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0196] Hereinafter, the present invention will be described using examples.
[0197] First, acid anhydrides, diamines, and solvents used in Examples are shown together with compound numbers or abbreviations below. In addition, in the examples, the abbreviation L represents the unit liter of capacity. Therefore, milliliters are expressed in mL.
[0198]
[0199] Anhydride (H-1)
[0200]
[0201] Anhydride (S-1)
[0202]
[0203] Anhydride (S-6)
[0204]
[0205] Anhydride (S-48)
[0206]
[0207]
[0208] Diamine (I-1)
[0209]
[0210] Diamine (I-2)
[0211]
[0212] Diamine (2-1-1)
[0213]
[0214] Diamine (2-1-66)
[0215]
[0216] Diamine (3-7-1) (Y 5 = n-pentyl)
[0217]
[0218] Diamine (4-1-4-1) (Y 6 = n-pentyl)
[0219]
[0220]
[0221] NMP: N-methyl-2-pyrrolidone
[0222] BC: Butyl cellosolve (ethylene glycol monobutyl ether)
Synthetic example 1
[0224]
[0225] According to Japanese Patent Laid-Open No. 2006-124371, 4-(3,5-dinitrobenzyl)phenol was synthesized. After mixing 10 g of this compound with 1 g of 5% palladium activated carbon in 100 mL of dimethylformamide, a hydrogenation reaction was carried out at 40° C. (hydrogen pressure: 680 kPa). After the reaction, the temperature was cooled to room temperature, the catalyst was removed by filtration, and the solvent was distilled off under reduced pressure to obtain the target product (7.5 g in yield, 81% in yield).
[0226] Melting point; 230.4°C-233.8°C
[0227] 1 H NMR; 9.15(s, 1H), 6.98(d, 2H, J=8.35Hz), 6.72(d, 2H, J=8.52Hz), 5.66(s, 3H), 4.63(br s, 4H)
Synthetic example 2
[0229]
[0230] 6.4 g (170 mmol) of commercially available sodium borohydride was added to a 2 L three-necked flask equipped with a thermometer, a stirrer, and a dropping funnel, and then 500 mL of dehydrated tetrahydrofuran was added. The solution was cooled to 5° C. or lower, and a solution obtained by dissolving 50 g (230 mmol) of commercially available 2,6-di-tert-butylbenzoquinone in 500 mL of dehydrated tetrahydrofuran was added dropwise thereto. The reaction liquid was warmed up to room temperature, and stirred at room temperature for 8 hours. After stirring, the obtained reaction liquid was poured into 1 L of 3M hydrochloric acid, and extracted with 1 L of ethyl acetate. After washing the organic layer twice with 1 L of pure water, anhydrous magnesium sulfate was added and dried. After filtering off anhydrous magnesium sulfate, the solvent was distilled off under reduced pressure to obtain 2,6-di-tert-butylhydroquinone (yield: 46 g, yield: 91%).
[0231]47 g (204 m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com