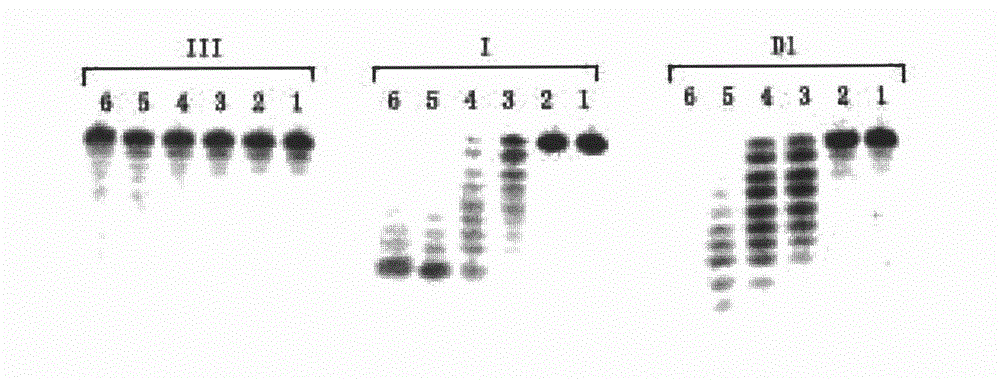
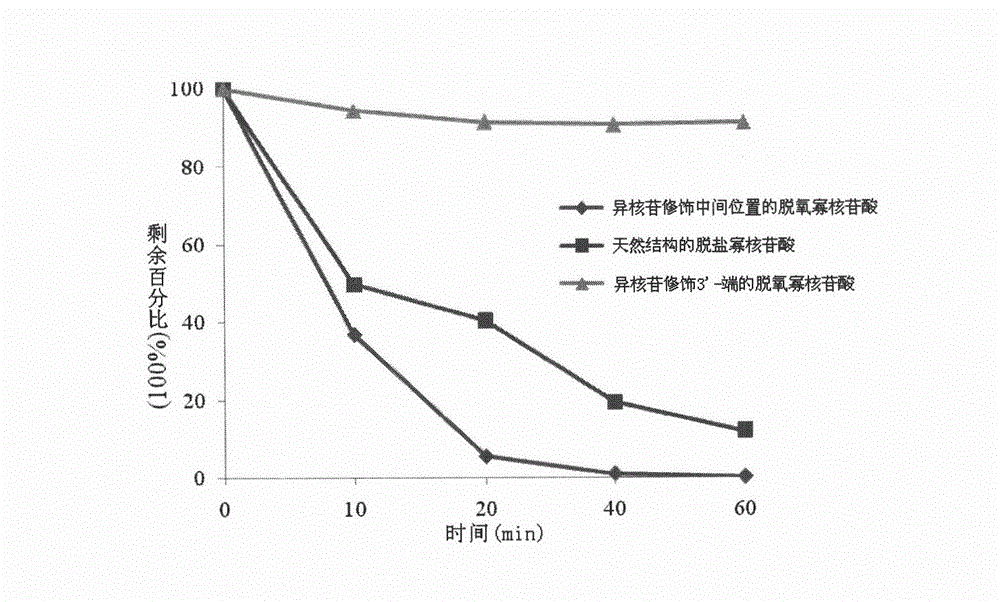
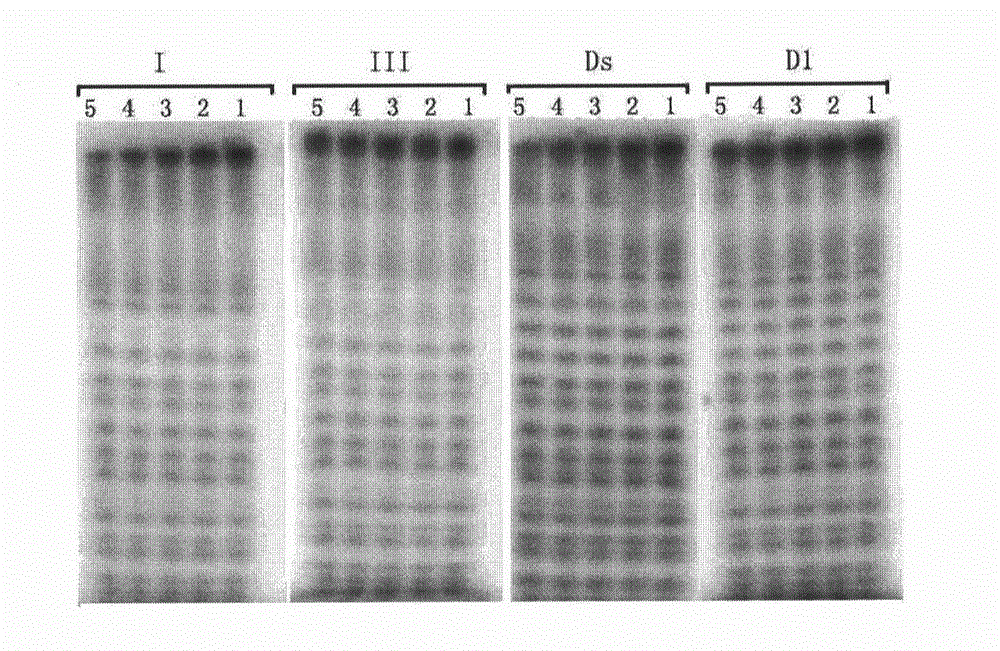
Isonucleoside compound or ortho-phosphite derivative thereof and preparation method and application thereof
A technology of isonucleoside compounds and compounds, applied in the application field of modifying siRNA and deoxy oligonucleotides, can solve the problems of complex chemical modification and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1 Synthesis of 5-(S)-hydroxymethylene-4-(R)-hydroxyl-3-(S)-(adeninyl-9')-tetrahydrofuran (n=1 in formula I, B is adenine)
[0085] 1.5-(R)-dimethoxymethyl-4-(R)-hydroxyl-3-(S)-(adeninyl-9')-tetrahydrofuran[5-(R)-dimethoxy-methyl-4-( Synthesis of R)-hydroxy-3-(S)-(adenyl-9')-tetrahydrofuran] (formula III)
[0086]Adenine (958mg, 7.09mmol), DBU (1.76ml, 11.79mmol) was placed in a microwave reaction flask, and the compound 5-(R)-dimethoxymethyl-3-(R), 4-(R )-epoxy-tetrahydrofuran (formula II, 942 mg, 5.88 mmol) in 20 ml of anhydrous DMF, capped, and stirred at room temperature for 10 min; the reaction bottle was placed in a microwave reactor, catalyzed by medium-power microwaves, and reacted for 30 min at 180 ° C; After suction filtration, the solvent was evaporated to dryness with an oil pump, and the column was separated under normal pressure to obtain 1.45 g of the product as a white solid with a yield of 83.5%.
[0087] 1 H NMR (300MHz, DMSO-d 6 ) δ3.30-3....
Embodiment 2
[0093] Example 2 Synthesis of [5S-(2-hydroxyethyl)-4R-hydroxyl-3S-(adeninyl-9-yl)]-tetrahydrofuran, [5S-(2-hydroxyethyl)-4R-hydroxyl-3S- Synthesis of (adenin-9-yl)-tetrahydrofuran (n=2 in formula I, B is adenine)
[0094] 1.(2R-dimethoxymethyl-3S-O-p-toluenesulfonyl-4R-O-benzoyl)-tetrahydrofuran[(2R-dimethoxymethyl-3S-O-p-toluenesulfonyl-4R-O-benzoyl)- tetrahydrofuran] (Formula IV)
[0095] (2R-Dimethoxymethyl-3S-O-p-toluenesulfonyl-4R-hydroxy)-tetrahydrofuran (1.99g, 5.99mmol) was dissolved in anhydrous pyridine (40mL), and BzCl (1.05mL, 9.11mmol) was added ) and DMAP (77 mg, 0.63 mmol). The reaction solution was stirred at room temperature overnight, the solvent was evaporated to dryness, the residue was dissolved in ethyl acetate, saturated NaHCO 3 solution and saturated NaCl solution, anhydrous Na 2 SO 4 Dry, filter and concentrate. Atmospheric pressure silica gel column separation, eluting with petroleum ether-ethyl acetate, gave light yellow syrup (2.56g, 98.1%). ...
Embodiment 3
[0126] Example 3 Synthesis of [5S-(2-hydroxypropyl)-4R-hydroxyl-3S-(adeninyl-9'-yl)]-tetrahydrofuran (n=3 in formula I, B is adenine)
[0127] 1. {2S-[2-E(Z)-ethoxycarbonylvinyl]-3R-O-p-toluenesulfonyl-4R-O-benzoyl}-tetrahydrofuran, {2S-[2-E(Z) -ethoxycarboxyl-vinyl]-3R-O-p-toluenesulfonyl-4R-O-benzoxy}-tetrahydrofuran} (Formula XIV)
[0128] (2R-Dimethoxymethyl-3S-O-p-toluenesulfonyl-4R-O-benzoyl)-tetrahydrofuran (3.00 g, 6.78 mmol) was dissolved in 87.5% aqueous trifluoroacetic acid (8 mL), React at room temperature for 5 hours, evaporate most of the solvent, and dissolve the residue with dichloromethane, then wash with saturated NaHCO 3 Wash with aqueous solution and saturated NaCl aqueous solution, dry over anhydrous MgSO4, filter, and concentrate to obtain white sugar bubbles. A suspension of NaH (60%, 275mg, 6.88mmol) in anhydrous THF (19mL) was kept at -30°C, and (EtO) was added dropwise 2 P(O)CH 2 COOEt (1.43mL, 6.99mmol), after stirring for about 30 minutes, added...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com