Preparation method of lithium bromide absorption cycle working fluid
An absorption cycle, lithium bromide technology, applied in chemical instruments and methods, heat exchange materials, etc., can solve the problems of difficult product separation and purification, by-products, low cycle performance coefficient, etc., to achieve easy product separation and purification. , easy operation, high purity and high quality results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
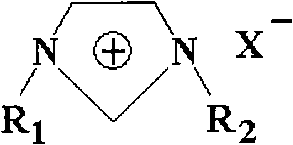
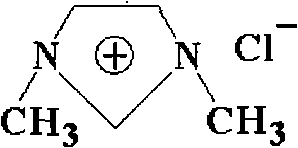
[0025] Embodiment 1: the synthesis of ionic liquid [DMIm]Cl
[0026] This example is an example of self-synthesis of ionic liquid [DMIm]Cl. Measure 100 mL of N-methylimidazole with a concentration >99% after vacuum distillation and place it in a vacuumized 500 mL reaction kettle. While stirring with a stirrer, CH 3 Cl to 0.6MPa, react at a temperature of 50-70°C for 24 hours. The reaction equation is as follows:
[0027]
[0028] After the reaction was finished, nitrogen gas was introduced, and then the remaining methylene chloride and nitrogen gas in the still were drained, and a white solid product was obtained after cooling, with a yield of 98%.
[0029] The product obtained above was recrystallized three times in acetonitrile solvent, and then vacuum-dried at a temperature of 110° C. for 24 hours. The dried product appears as colorless or light yellow crystals at 25°C.
[0030] H NMR spectroscopy ( 1 HNMR) to characterize the structure of the product, the product ...
Embodiment 2
[0031] Embodiment 2: Add the thermophysical property change of ionic liquid [DMIm]Cl in lithium bromide aqueous solution
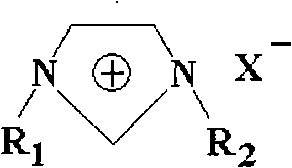
[0032] At room temperature, with the DMIm]Cl prepared in Example 1, according to the mass ratio of LiBr to [DMIm]Cl is 3:1, a saturated solution is prepared, the concentration mass fraction of solute (LiBr and [DMIm]Cl) is 69%, the boiling point It is 164.0°C. And at the same temperature, the boiling point of a saturated lithium bromide solution with a solute mass fraction of 60% is 155.5°C. Compared with the two, the boiling point of the new working fluid increases by 8.5°C, and the solubility increases by 15.0%. An increase in the boiling point of the system means a decrease in the vapor pressure of the system, and the solution at this time has relatively strong absorption characteristics for water vapor. The higher the concentration of the solution, the higher the boiling point rises, but the crystallization temperature limits the concentration of the...
Embodiment 3
[0033] At room temperature, according to LiBr vs. [DMIm]BF 4 The mass ratio is 4:1, and a saturated solution is prepared with a concentration mass fraction of 78% and a boiling point of 161.5°C. And at the same temperature, the boiling point of a lithium bromide saturated solution with a concentration mass fraction of 60% is 155.5°C. Compared with the two, the boiling point increased by 6.0°C and the solubility increased by 30.0%. Embodiment 4: Add the thermophysical property change of ionic liquid [EMIm]Br in lithium bromide aqueous solution
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com