Preparation carried with particles of antitumor drug and preparation method thereof
A technology of anti-tumor drugs and preparations, which is applied in the field of preparations loaded with anti-tumor drug particles, which can solve the problems of necrosis of mesenchymal stem cells, achieve the effects of improving curative effect, broad application prospects, and reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
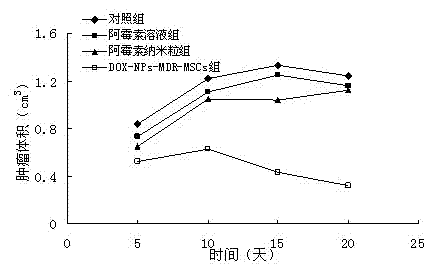
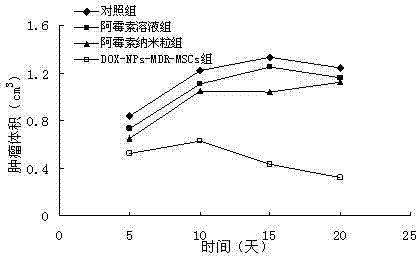
[0032] Example 1 Preparation of Adriamycin Hydrochloride Microparticles
[0033] a. Preparation of anti-tumor drug microparticles: prepared by emulsification-solvent evaporation method. The preparation process is as follows: doxorubicin hydrochloride and PLA are dissolved in DMSO to form an organic phase. The above organic phase was emulsified by ultrasound in pH 7.4 phosphate buffer solution containing PVA205, and the organic solvent was evaporated quickly by drying under reduced pressure to prepare doxorubicin hydrochloride PLA with an average particle size of 120nm and a drug content of 2.5%. particle.
[0034] b. Preparation of multidrug-resistant mesenchymal stem cells expressing P-glycoprotein: using retroviruses as vectors, transfecting multidrug-resistant genes into mesenchymal stem cells to obtain multidrug-resistant mesenchymal stem cells expressing P-glycoprotein Stem cells, spare.
[0035] The preparation process is as follows: use liposome transfection method t...
Embodiment 2
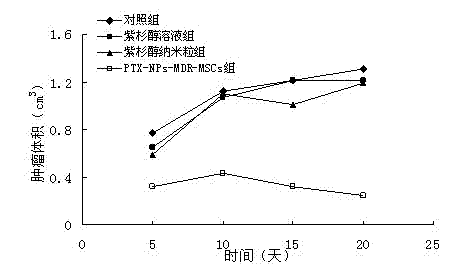
[0037] Example 2 Preparation of paclitaxel-loaded microparticles
[0038] a. Preparation of anti-tumor drug microparticles: prepared by emulsification-solvent evaporation method. The preparation process is as follows: paclitaxel and PLA are dissolved in dichloromethane to form an organic phase. The above organic phase was emulsified by ultrasound in a pH 7.4 phosphate buffer containing Tween 80, and the organic solvent was quickly evaporated by drying under reduced pressure to prepare paclitaxel PLA particles with an average particle size of 95nm and a drug content of 4.1%.
[0039] b. Preparation of multidrug-resistant mesenchymal stem cells expressing P-glycoprotein: using lentivirus as a carrier, transfecting multidrug-resistant genes into mesenchymal stem cells to obtain multidrug-resistant mesenchymal stem cells expressing P-glycoprotein Stem cells, spare; the preparation process is as follows: import pMX-mdr1-GFP and outer membrane protein particles PCI VSV and PCIPGB i...
Embodiment 3
[0041] Example 3 Formulations Carrying Docetaxel Microparticles
[0042] a. Preparation of anti-tumor drug microparticles: prepared by emulsification-solvent evaporation method. The preparation process is as follows: docetaxel and PLGA are dissolved in acetone to form an organic phase. The above organic phase was emulsified by ultrasound in pH 7.4 phosphate buffer solution containing poloxamer 188, and the organic solvent was quickly evaporated by drying under reduced pressure to prepare polysaccharides with an average particle size of 80 nm and a drug content of 0.01%. Paclitaxel PLGA microparticles, wherein the molar ratio of propiolactone to betaine in PLGA is 1 / 99;
[0043] b. Preparation of multidrug-resistant mesenchymal stem cells expressing P-glycoprotein: using retroviruses as vectors, transfecting multidrug-resistant genes into mesenchymal stem cells to obtain multidrug-resistant mesenchymal stem cells expressing P-glycoprotein Mesenchymal stem cells, spare;
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com