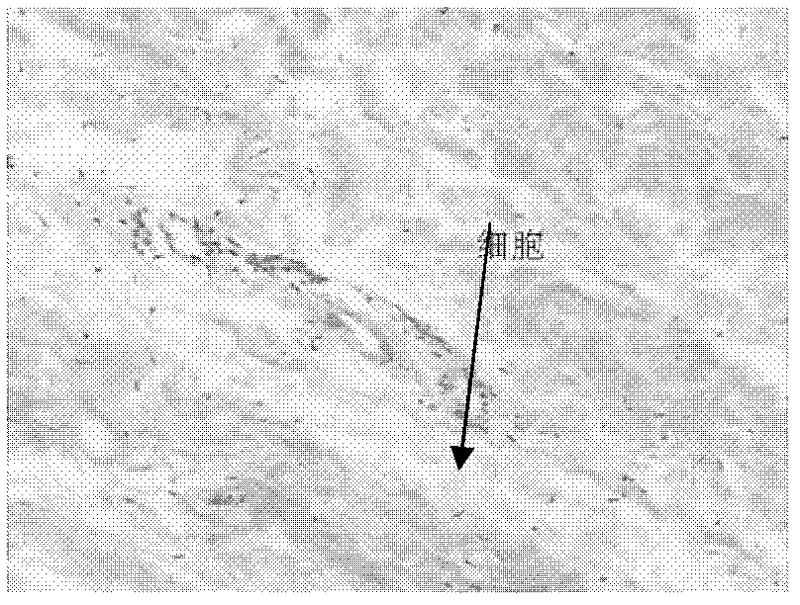
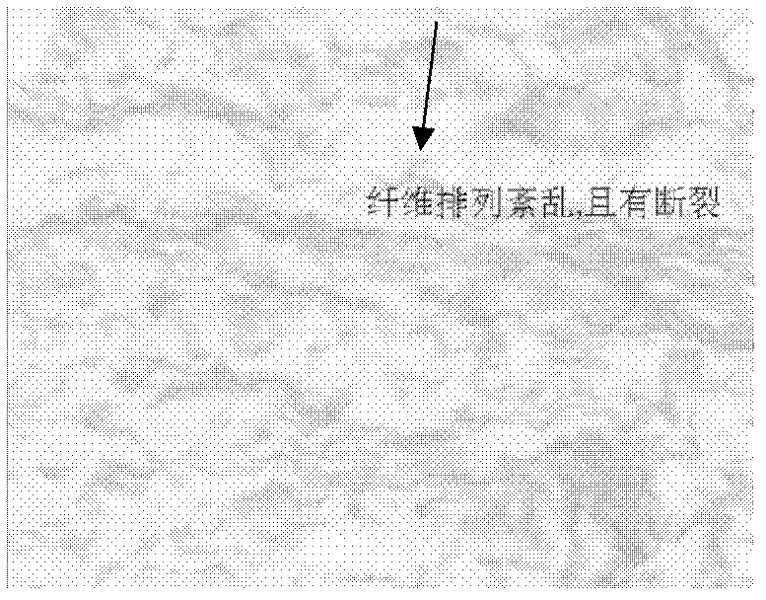
Preparation method of acellular dried active amnion
A decellularization and amniotic membrane technology, applied in the field of medical materials, can solve the problems of strong destructive effect, fiber damage, loose and disordered fiber arrangement, and achieve the effects of no cytotoxicity, maintaining integrity, and good mechanical properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A preparation method of decellularized dry active amnion, comprising the steps of:
[0027] (1) Raw material processing: Take the normal full-term human placenta after cesarean section that has passed the pathogen detection, wash it with sterile saline, remove the blood components, bluntly separate the amniotic membrane from the placental chorion, and scrape it with a cell curette Remove the matrix components attached to the amnion matrix surface;
[0028] (2) Repeated freezing and thawing: the amniotic membrane treated in step (1) was frozen at -70°C for 4 hours, then placed in a water bath at 37°C for 20 minutes, and this was repeated 3 times.
[0029] (3) Cleaning: Immerse the amniotic membrane treated in step (2) in PBS solution, wash it on a shaker at 4°C for 24 hours, take it out and immerse it in detergent, and shake it for 24 hours at 30°C at a speed of 100 rpm on a shaker ; Take it out, then immerse it in PBS solution, and shake it at 8°C for 48 hours at a spe...
Embodiment 2
[0034] A preparation method of decellularized dry active amnion, comprising the steps of:
[0035] (1) Raw material processing: Take the normal full-term human placenta after cesarean section that has passed the pathogen detection, wash it with sterile saline, remove the blood components, bluntly separate the amniotic membrane from the placental chorion, and scrape it with a cell curette Remove the matrix components attached to the amnion matrix surface;
[0036] (2) Freezing and thawing: freeze the amniotic membrane treated in step (1) at -60°C for 6 hours, and then place it in a water bath at 37°C for 40 minutes;
[0037] (3) Cleaning: Immerse the amniotic membrane treated in step (2) in PBS solution, wash it on a shaker at 4°C for 18 hours, take it out and immerse it in detergent, shake it at 40°C for 18 hours at a speed of 20 rpm on a shaker ; Take it out, then immerse it in PBS solution, and shake it at 10°C for 56 hours at a speed of 20 rpm on a shaker; change the PBS s...
Embodiment 3
[0042] A preparation method of decellularized dry active amnion, comprising the steps of:
[0043] (1) Raw material processing: Take the normal full-term human placenta after cesarean section that has passed the pathogen detection, wash it with sterile saline, remove the blood components, bluntly separate the amniotic membrane from the placental chorion, and scrape it with a cell curette Remove the matrix components attached to the amnion matrix surface;
[0044] (2) Repeated freezing and thawing: the amniotic membrane treated in step (1) was frozen at -80°C for 2 hours, then placed in a water bath at 37°C for 10 minutes, and repeated 5 times;
[0045] (3) Cleaning: Immerse the amniotic membrane treated in step (2) in PBS solution, wash it on a shaker at 4°C for 30 hours, take it out and immerse it in detergent, shake it at 20°C for 30 hours at a speed of 200 rpm on a shaker ; Take it out, then immerse it in PBS solution, and shake it at 2°C for 40 hours at a speed of 200 rpm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com