Fluorescent polymerase chain reaction (PCR) primer, probe and method for detecting A, C and G group of hemolytic streptococcus
A hemolytic streptococcus and probe technology, which is applied in the fields of probes, detection of the whole group of hemolytic streptococci, and fluorescent PCR primers, can solve the problems of high experience requirements for inspectors, cumbersome detection steps, long detection cycle, etc., and achieve The effect of wide detection range, short detection time and large detection amount
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Design and synthesis of primers and probes.
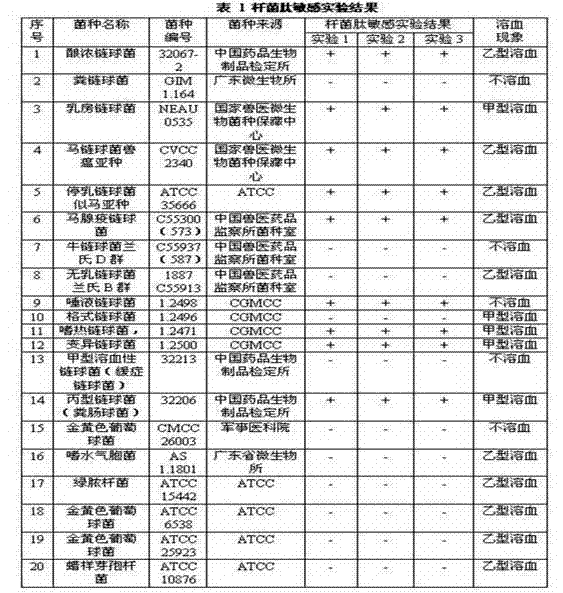
[0034] The streptococci with beta hemolytic characteristics are mainly group A, B, C and G streptococci, while the streptococci with streptokinase-positive characteristics are group A, C and G streptococci, and the streptococci with bacitracin-positive characteristics are No related reports were found in the literature for which groups, so bacitracin sensitivity experiments were carried out.
[0035] The results are shown in Table 1. If the diameter of the bacitracin disk inhibition zone is greater than 10 mm, it is judged as bacitracin-sensitive, that is, the bacitracin-sensitive test is positive, and is indicated by +; if the bacitracin disk inhibition zone is less than or equal to 10 mm, it is judged as bacitracin-resistant The bacitracin sensitivity test was negative, indicated by -. The results showed that the strains with positive bacitracin test and B hemolytic characteristics were group A, C and G hemoly...
Embodiment 2
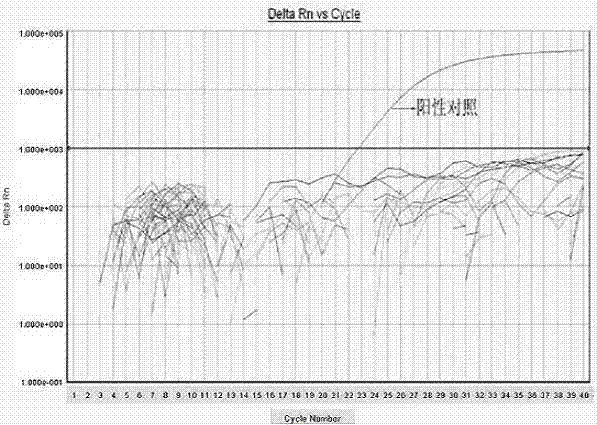
[0045] Example 2 Fluorescent PCR detection method.
[0046] Aseptic procedures were performed in a biological safety cabinet.
[0047] Sample processing: Sample dilution and enrichment were performed in a P2 clean room according to the requirements of GB / T 4789.11-2003, and the sample enrichment solution was placed in an incubator at 37°C for 18 hours.
[0048] DNA template: Take 1mL of enrichment solution in a 1.5mL centrifuge tube, centrifuge at 12000rpm for 10min, remove the supernatant, and collect the bacteria. Add 100 μL of sterilized ultrapure water, mix well, and bathe in boiling water at 100°C for 10 minutes. Centrifuge at 12000rpm for 10min, and take the supernatant as the reaction template.
[0049] Reaction system: forward primer (20 μmol / L), 0.4 μL; reverse primer (20 μmol / L), 0.4 μL; probe (20 μmol / L), 0.2 μL; Premix Ex Taq (2×), 10 μL; DNA template, 5uL; add sterilized ultrapure water to make up the reaction system to 20 μL. The 5' end of the probe is lab...
Embodiment 3
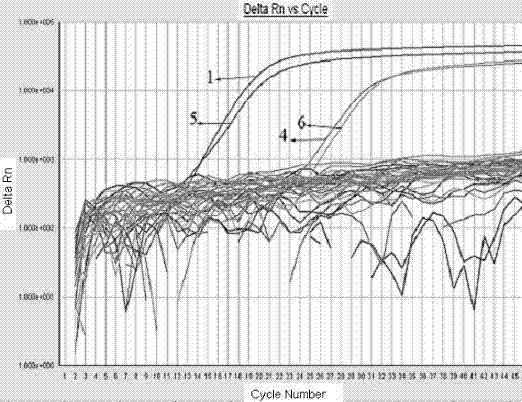
[0052] Example 3 Specificity of detection of hemolytic streptococcus by fluorescent PCR.
[0053] According to the fluorescent PCR primers, probes and methods provided by the present invention, 35 common foodborne pathogenic bacteria (Table 2) were amplified to analyze the specificity of the fluorescent PCR detection method. Streptococcus pyogenes (No. 1), Streptococcus equi subsp. zooepidemicus (No. 4), Streptococcus dysgalactiae subsp. equisimilis (No. 5) and Streptococcus equine epidemics (No. 6) according to GB / T 4789.11-2003 Confirmed as hemolytic streptococci. Streptococcus pyogenes (No. 1), Streptococcus equi subsp. zooepidemicus (No. 4), Streptococcus dysgalactiae subsp. Streptococcus (No. 2), Streptococcus uberis (No. 3) and Streptococcus bovis group D (No. 7) were used as negative controls, and sterilized ultrapure water was used as a blank control. Two parallels were set up for each test group.
[0054] CMCC is the abbreviation of China Medical Microbiology Cult...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com