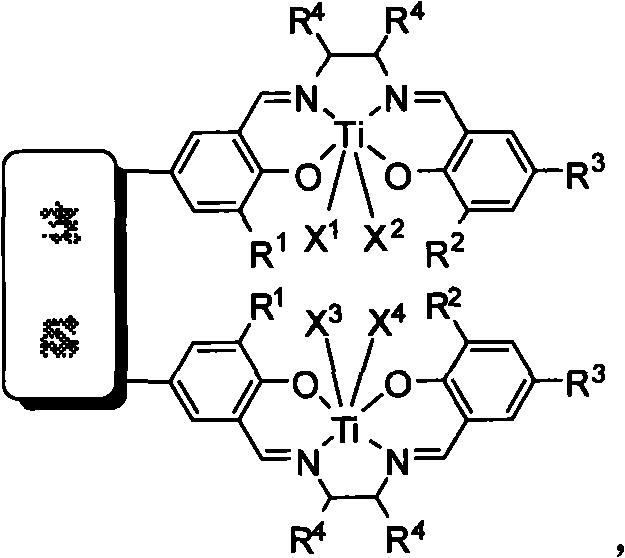
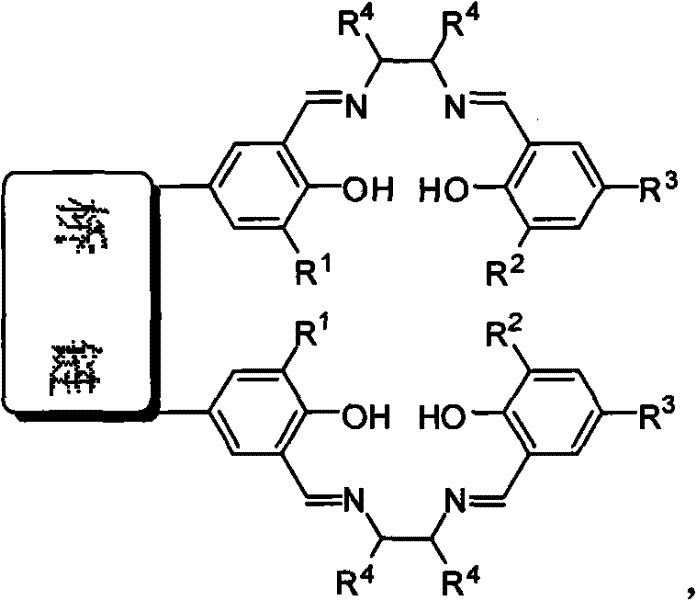
Bridged bis-Schiff-base-titanium complex, and synthesis method and application thereof
A double Schiff base, titanium complex technology, applied in titanium organic compounds, asymmetric synthesis, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problems of reduced catalytic activity and enantioselectivity , to achieve the effect of improving activity and enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the synthesis of double Salen ligand 7a
[0041] 1) Synthesis of compound 3a from compounds 1a and 2a
[0042] Add compound 1a (775mg, 4.0mmol, synthetic method reference to [Jacobsen, E.N.et al., J.Am.Chem.Soc.2001, 123, 2687]) and cis-5-norbornene to a 50mL three-necked flask -endo-2,3-dicarboxylic acid (363mg, 2.0mmol) and DMAP (49mg, 0.4mmol), add dichloromethane (8mL) and DMF (0.8mL) after pumping out argon, stir for 10 minutes and cool to 0°C, add DIC (0.53g, 4.2mmol) and keep stirring at 0°C for 10 minutes, rise to room temperature and stir for 72 hours, TLC detects that the reaction is complete, add dichloromethane (50mL) to the reaction solution, add 0.1M hydrochloric acid (20mL) Washing, saturated aqueous NaCl washing (3 × 30mL), anhydrous NaCl 2 SO 4 After drying, it was filtered, and the solvent was removed under reduced pressure. It was separated by column chromatography (petroleum ether: ethyl acetate = 8:1) to obtain 0.816 g of a white so...
Embodiment 2
[0047] Embodiment 2: the synthesis of compound 7b
[0048] Add dropwise the chloroform solution (800mg, 1.44mmol, in 20mL) of 3b (synthetic method is the same as 3a) to the solution of 6a, rise to room temperature after the dropwise addition, add Molecular sieves were stirred for 48 hours, filtered and washed with dichloromethane, the solvent was removed under reduced pressure, and column chromatography (petroleum ether: ethyl acetate = 20:1) gave 320 mg of a yellow foamy solid. Yield 63%; m.p.169-170°C; [α] D 20 =-231.7 (c=0.51, CHCl 3 ); 1 H NMR (300MHz, CDCl 3 )δ1.26(s, 18H), 1.41(s, 18H), 1.44(s, 18H), 1.47-1.52(m, 3H), 1.71-1.78(m, 5H), 1.89-2.00(m, 8H) , 3.35-3.38(m, 4H), 6.94(d, 2H, J=2.7Hz), 7.01(d, 2H, J=2.4Hz), 7.10(d, 2H, J=2.7Hz), 7.34(d, 2H, J=2.4Hz), 7.66(t, 1H, J=7.8Hz), 8.29(s, 2H), 8.34(s, 2H), 8.42(dd, 2H, J=7.6, 1.6Hz), 8.95( s, 1H), 13.65 (br, 2H), 13.99 (br, 2H) ppm; 13 C NMR (75MHz, CDCl 3 )δ 24.2, 29.1, 29.4, 31.4, 33.1, 33.2, 34.0, 34.9, 72....
Embodiment 3
[0049] Embodiment 3: the synthesis of compound 7c
[0050] Add dropwise the chloroform solution (1.2g in 30mL) of 3c (synthetic method is the same as 3a) to the solution of 6a, rise to room temperature after the dropwise addition, add Molecular sieves were stirred for 48 hours, filtered and washed with dichloromethane, the solvent was removed under reduced pressure, and column chromatography (petroleum ether: ethyl acetate = 25:1) gave 380 mg of a yellow foamy solid. Yield 37%; m.p. 170-172°C; [α] D 20 =-204.1 (c=0.50, CHCl 3 ); 1 H NMR (300MHz, CDCl 3 )δ1.19(s, 18H), 1.30(s, 18H), 1.34(s, 18H), 1.41-1.48(m, 6H), 1.67(s, 6H), 1.89-2.05(m, 10H), 3.28- 3.30(m, 4H), 6.71(d, 2H, J=2.7Hz), 6.89(d, 2H, J=2.1Hz), 7.03(d, 2H, J=2.1Hz), 7.19(t, 2H, J =7.9Hz), 7.26(d, 2H, J=3.0Hz), 7.61(dd, 2H, J=8.1, 1.2Hz), 7.74(dd, 2H, 7.8, 1.5Hz), 7.91(s, 2H), 8.27 (s, 2H), 13.56 (br, 2H), 13.89 (br, 2H) ppm; 13 C NMR (75MHz, CDCl 3 )δ24.2, 29.1, 29.3, 31.3, 31.6, 32.8, 33.0, 33.9, 34.4,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com