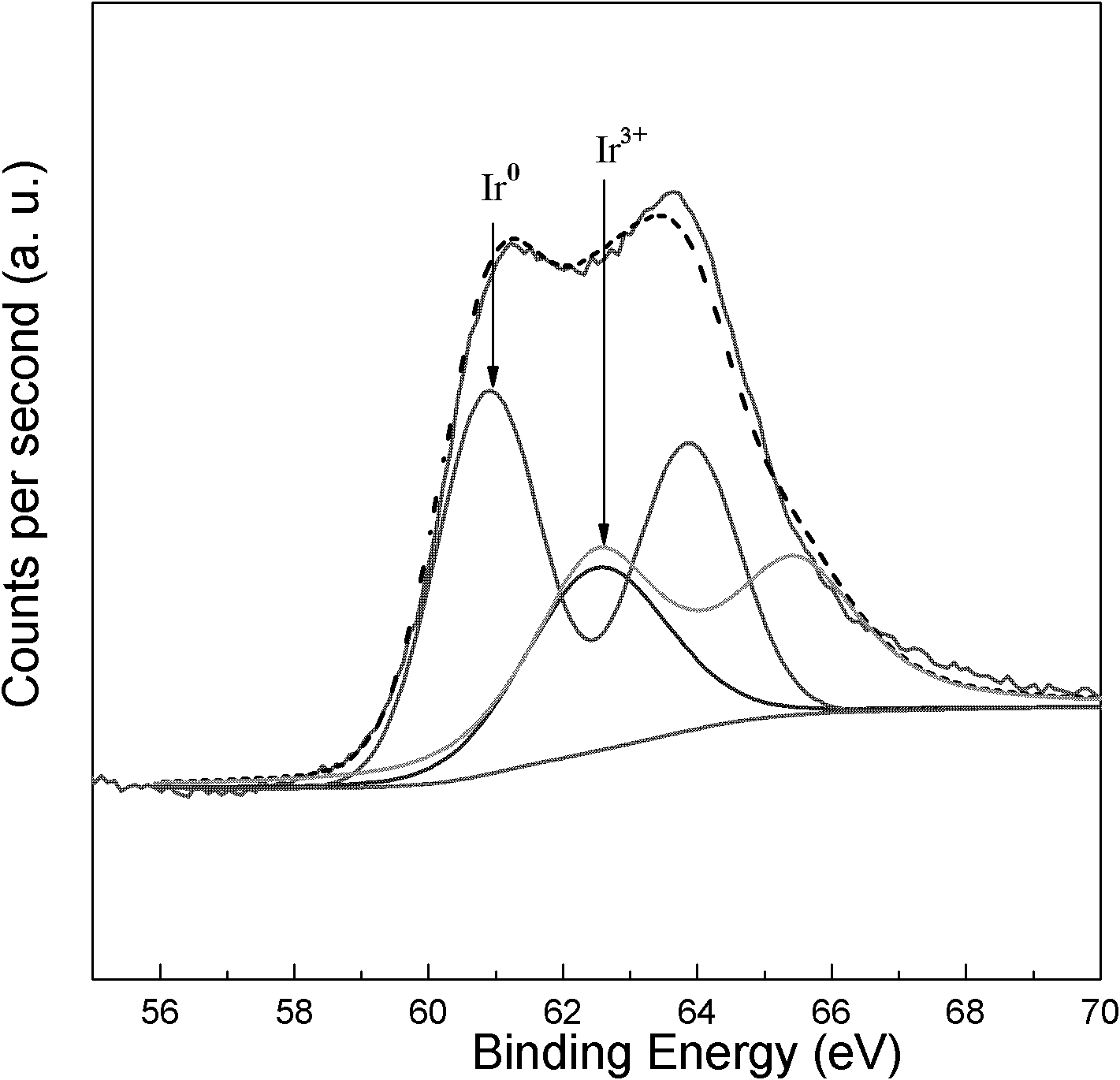
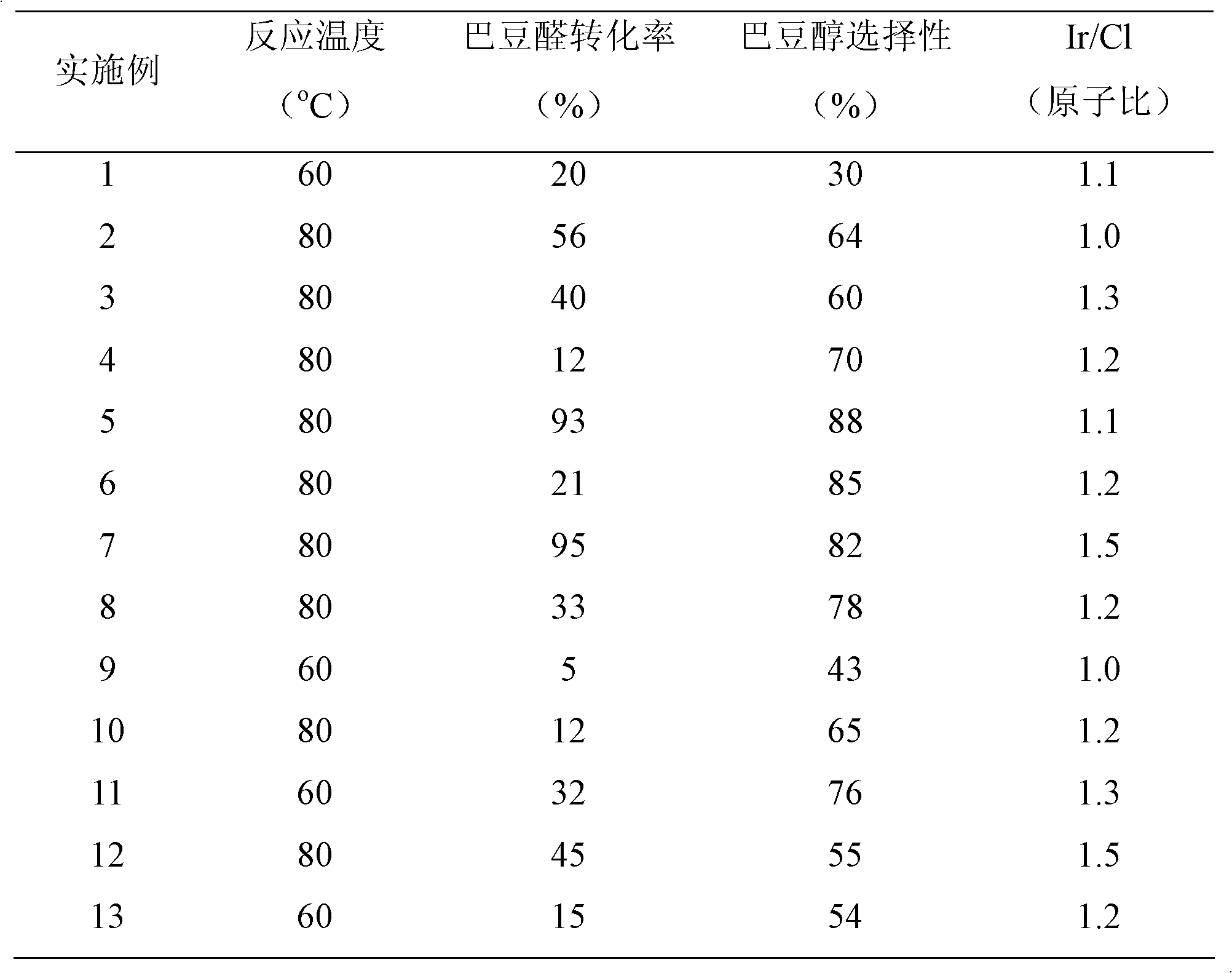
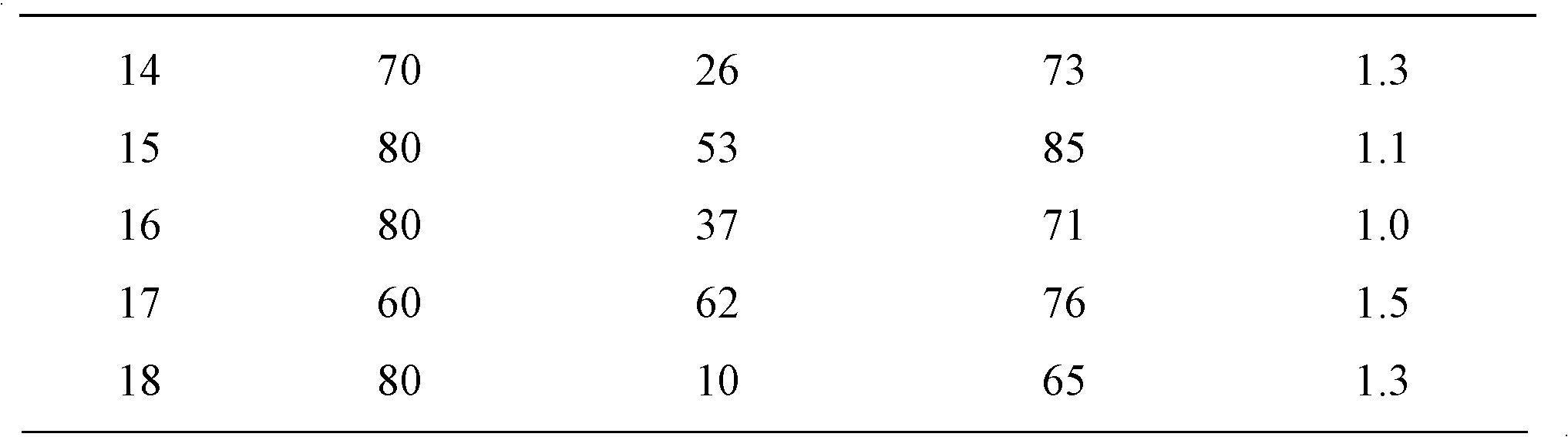
Catalyst for preparing crotyl alcohol from gas-phase crotonaldehyde through selective hydrogenation and preparation method thereof
A crotonaldehyde and selectivity technology, which is applied to the catalyst and preparation field for the selective hydrogenation of gas-phase crotonaldehyde to crotyl alcohol, and can solve the problems of low selectivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] ①Dry TiO in vacuum at 100℃ 2 carrier;
[0022] ② Prepare H at a concentration of 0.007g / ml 2 IrCl 6 solution. Take 0.72ml of H 2 IrCl 6 Add 1gTiO to the solution 2 In the carrier, after stirring evenly, soak at room temperature for 12 hours;
[0023] ③ Then evaporate the liquid to dryness in a water bath at 90°C, and dry at 100°C in a nitrogen atmosphere for 12 hours to obtain a solid powder;
[0024] ④The solid powder in ③ is heated up to 200°C in hydrogen and subjected to reduction treatment for 1 hour, and then the catalyst of the present invention is obtained, wherein the mass percentage of Ir is 0.5%, and Ir is loaded on the carrier TiO 2 superior.
[0025] The test of crotonaldehyde hydrogenation activity is shown in Table 1: under normal pressure, it was inspected in a fixed-bed reactor, the amount of catalyst was 0.2g, the volume ratio of hydrogen / crotonaldehyde was 94:1, the total flow rate was 26ml / min, and the reaction temperature was 60°C .
Embodiment 2
[0027] ①Dry TiO in vacuum at 100℃ 2 carrier;
[0028] ② Prepare H at a concentration of 0.007g / ml 2 IrCl 6 solution. Take 0.72ml of H 2 IrCl 6 Add 1gTiO to the solution 2 In the carrier, after stirring evenly, soak at room temperature for 12 hours;
[0029] ③ Then evaporate the liquid to dryness in a water bath at 90°C, and dry at 100°C in a nitrogen atmosphere for 12 hours to obtain a solid powder;
[0030] ④ heat up the solid powder in ③ to 300° C. for reduction treatment in hydrogen for 1 hour, and then obtain the catalyst of the present invention, wherein the mass percentage of Ir is 0.5%, and Ir is loaded on the carrier TiO 2 superior.
[0031] The test of crotonaldehyde hydrogenation activity is shown in Table 1: under normal pressure, it was inspected in a fixed-bed reactor, the amount of catalyst was 0.2g, the volume ratio of hydrogen / crotonaldehyde was 94:1, the total flow rate was 26ml / min, and the reaction temperature was 80°C .
Embodiment 3
[0033] ①Dry TiO in vacuum at 100℃ 2 carrier;
[0034] ② Prepare H at a concentration of 0.007g / ml 2 IrCl 6 solution. Take 1.44ml of H 2 IrCl 6 Add 1gTiO to the solution 2 In the carrier, after stirring evenly, soak at room temperature for 12 hours;
[0035] ③ Then evaporate the liquid to dryness in a water bath at 90°C, and dry at 100°C in a nitrogen atmosphere for 12 hours to obtain a solid powder;
[0036] ④The solid powder in ③ is heated up to 200°C in hydrogen and subjected to reduction treatment for 1 hour, and then the catalyst of the present invention is obtained, wherein the mass percentage of Ir is 1%, and Ir is loaded on the carrier TiO 2 superior.
[0037] The test of crotonaldehyde hydrogenation activity is shown in Table 1: under normal pressure, it was inspected in a fixed-bed reactor, the amount of catalyst was 0.2g, the volume ratio of hydrogen / crotonaldehyde was 94:1, the total flow rate was 26ml / min, and the reaction temperature was 80°C .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com