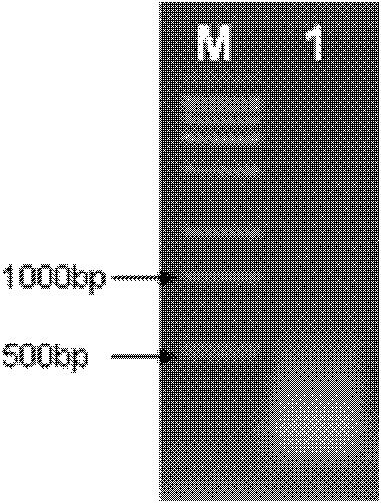PPA-linker-Thanatin fusion protein and preparation method thereof
A technology of fusion protein and protein, applied in the direction of hybrid peptide, recombinant DNA technology, chemical instruments and methods, etc., can solve the problem of low expression activity of fusion protein, and achieve the effect of development and application prospects of major new drug markets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1. Obtaining of the cDNA gene of Pinellia palmata lectin
[0039] Total RNA was extracted from the tuber of Pinellia pedatisecta Schott. According to the TransScript First-Strand cDNA Synthesis SuperMix instruction manual, the total RNA of Pinellia pedatisecta Schott was reverse-transcribed into single-stranded cDNA.
[0040] The synthesis system of First-Strand cDNA is as follows:
[0041]
[0042] The temperature of the PCR instrument was controlled at 50°C for 30 minutes; at 85°C for 5 minutes, the RT Enzyme was inactivated. cDNA after electrophoresis ( figure 1 ) shows that the cDNA is a diffuse band below 1 kb, which is in line with the expected result. Using cDNA as a template, two pairs of primers PPAL1 and PPAL2 were designed according to the complete sequence of Pinellia pedatisecta agglutinin (PPA) mRNA reported on NCBI (GeneBank: HM593586.1), respectively:
[0043]
[0044] Among them, PPAL1 and PPAL2 were respectively introduced into restric...
Embodiment 2
[0051] The cloning of embodiment 2, ppa-linker-thanatin recombinant gene
[0052] The gene ppa encoding Pinellia palmata lectin was amplified by three-step PCR with primers P1, P2, P3, and P4, and the recombinant gene ppa-linker-thanatin was amplified. The overlapping genes are connected to the 5' end of the ppa ORF through P1, P2, and P3, respectively. The PCR products of each step were purified by tapping and cloned into the T vector, and after identification by plasmid PCR and enzyme digestion, Sangon was commissioned for sequencing.
[0053]
[0054] The PCR system is as follows:
[0055]
[0056]
[0057] P1, P4 product (ppal1, primers P1, P4, annealing temperature 55 degrees), P2, P4 product (ppal2, primers P2, P4, annealing temperature 65 degrees), P3, P4 product (ppal3, primers P3, P4, annealing temperature 56 degrees) were respectively connected to the pEasy T1 carrier, and the connection system was the same as in Example 1, identified by PCR and enzyme dig...
Embodiment 3
[0060] Embodiment 3, the construction of ppa-linker-thanatin prokaryotic expression vector
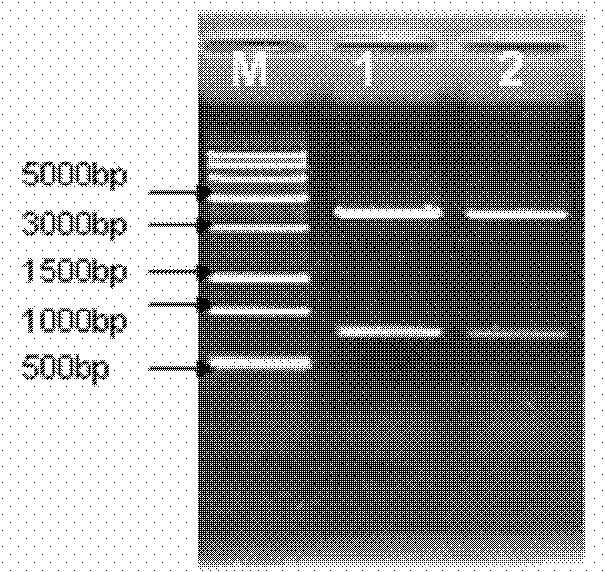
[0061] ppa-linker-thanatin (pt) gene and pET28a gene were digested by SalI and XhoI, and the target fragment was recovered. Under the action of T4 DNA ligase, the expression vector pET28a-pt was constructed, and the vector was transformed into T1 competent cells, at 37°C Plasmids were extracted after culturing for 12 hours, identified by PCR and enzyme digestion ( Figure 9 ) shows that the vector was constructed successfully.
[0062] The amino acid sequence corresponding to the expected recombinant gene is shown in SEQ ID NO: 2 (ie, the second item in the sequence listing).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com