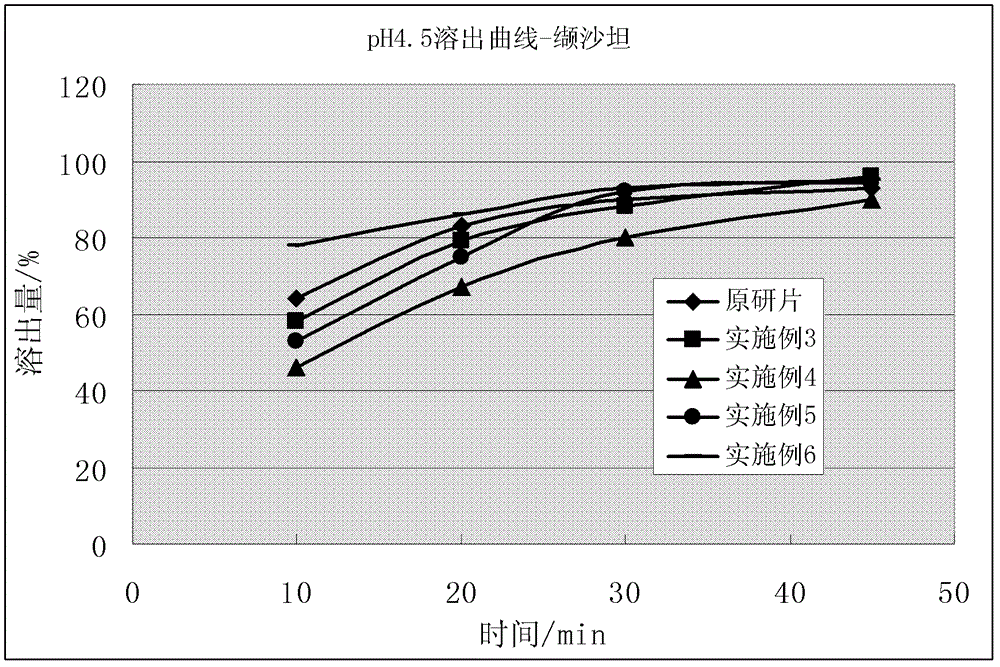
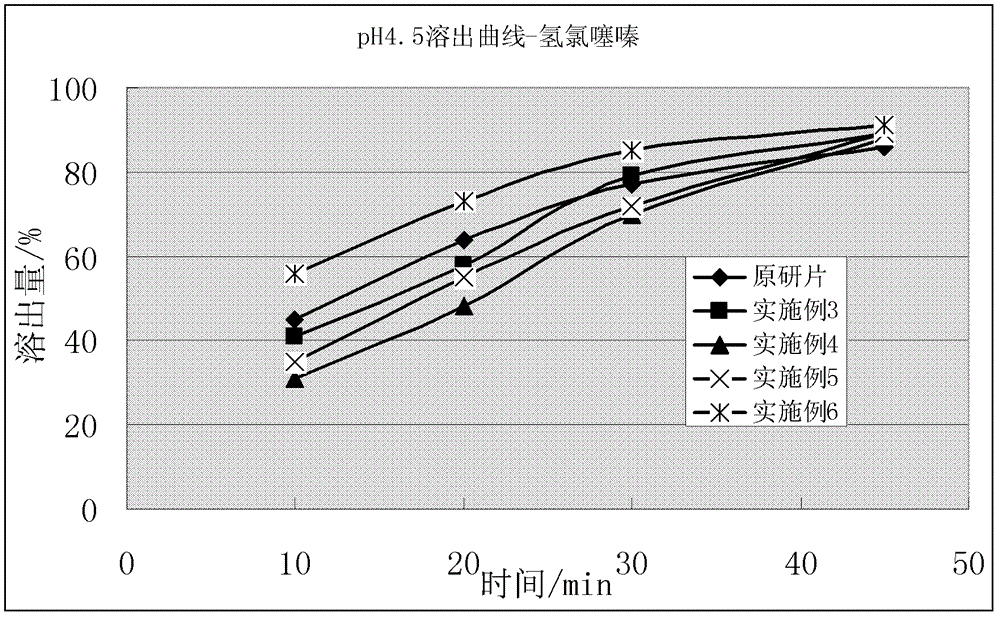
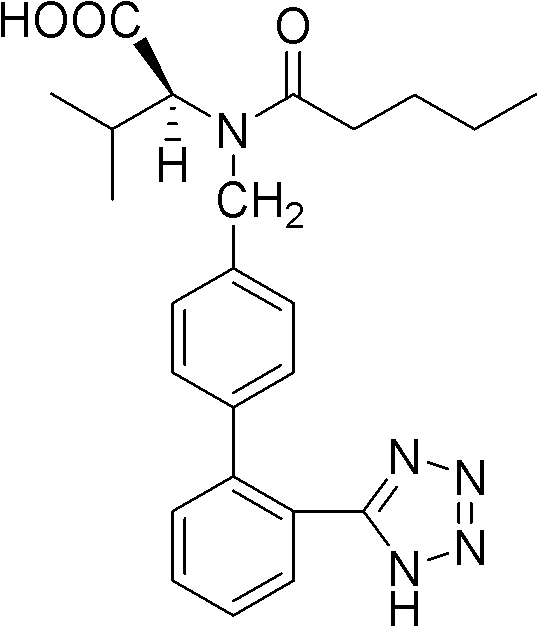
Compound solid preparation of valsartan and hydrochlorothiazide, and preparation method thereof
A technology of tan hydrochlorothiazide and solid preparations, which is applied in the field of medicine, can solve problems such as the dissolution of valsartan, and achieve the effects of good industrial production value, high dissolution rate, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The prescription is (specification 80mg / 12.5, per 1000 tablets);
[0052] A: Valsartan 80g
[0053] B: Hydrochlorothiazide 12.5g
[0054] C: Microcrystalline cellulose 158.3g
[0055] D: Crospovidone 15g
[0056] E: micronized silica gel 1.5g
[0057] F: Magnesium stearate 2.7g
[0058] Materials A, B, C and D were passed through a 30-mesh sieve, and mixed uniformly to obtain mixture I; silicon dioxide and magnesium stearate were added to mixture I, and mixed uniformly to obtain mixture II, and the measured angle of repose was 43.7°. It is not suitable for direct compression of dry powder; Sinopharm Longli ZP-10 Rotary Tablet Press hopper feeding is not smooth, tablet cannot be pressed smoothly, and the difference in tablet weight is greater than 7.5%.
[0059] The powder with a tablet weight of 270 mg was manually weighed, and the dissolution rate was measured after tablet compression (1000 ml dissolution medium, paddle method 50 rpm). The results show that the d...
Embodiment 2
[0061] The prescription is (specification 80mg / 12.5, per 1000 tablets);
[0062] A: Valsartan 80g
[0063] B: Hydrochlorothiazide 12.5g
[0064] C: Microcrystalline Cellulose 150.2g 55.6%
[0065] D: Partially pregelatinized starch 8.1g 3%
[0066] E: Crospovidone 15g
[0067] F: Micropowder silica gel 1.5g
[0068] G: Magnesium stearate 2.7g
[0069] Materials A, B, C and D were passed through a 30-mesh sieve, and mixed uniformly to obtain mixture I; silicon dioxide and magnesium stearate were added to mixture I, and mixed uniformly to obtain mixture II, and the measured angle of repose was 44.8°. It is not suitable for direct compression of dry powder; Sinopharm Longli ZP-10 rotary tablet press does not discharge smoothly from the hopper and cannot press tablets smoothly. Tablet weight differences were greater than 7.5%.
[0070] The powder with a tablet weight of 270 mg was manually weighed, and the dissolution rate was measured after tablet compression (1000 ml diss...
Embodiment 3
[0071] Embodiment 3, tablet of the present invention
[0072] The prescription is (specification 80mg / 12.5, per 1000 tablets);
[0073] A: Valsartan 80g
[0074] B: Micropowder silica gel 1.5g
[0075] C: Hydrochlorothiazide 12.5g
[0076] D: Microcrystalline cellulose 150.2g
[0077] E: Partially pregelatinized starch 8.1g
[0078] F: Crospovidone 15g
[0079] G: Magnesium stearate 2.7g
[0080] Pass materials A and B through a 30-mesh sieve, and mix well to obtain mixture I; add half of the amount of microcrystalline cellulose to mixture I and mix for about 5 minutes, then add the remaining microcrystalline cellulose, partially pregelatinized starch and hydrochlorothiazide Mix for about 5 minutes to obtain mixture II, finally add magnesium stearate into mixture II, mix evenly to obtain mixture III, measure the angle of repose to be 40.3°, and feed smoothly in the hopper of Sinopharm Longli ZP-10 rotary tablet press, The tableting went well. Tablet weight variance was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com