Spherical nitrogen-doped carbon-supported non-noble metal oxygen reduction catalyst and preparation method thereof
A non-precious metal, nitrogen-doped carbon technology, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve unfavorable catalyst industrialization, difficult control of preparation process conditions, and complex catalyst preparation methods and other problems to achieve the effect of avoiding the loss of positive electrode potential, low price and improving service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
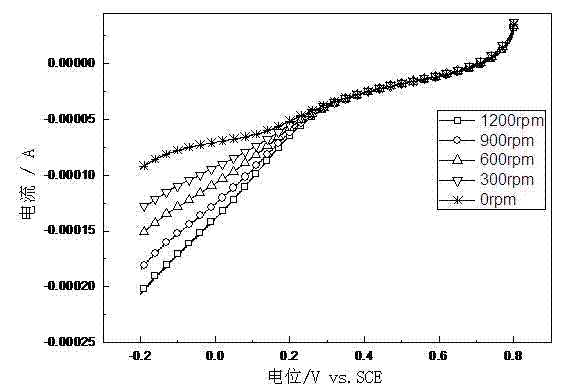
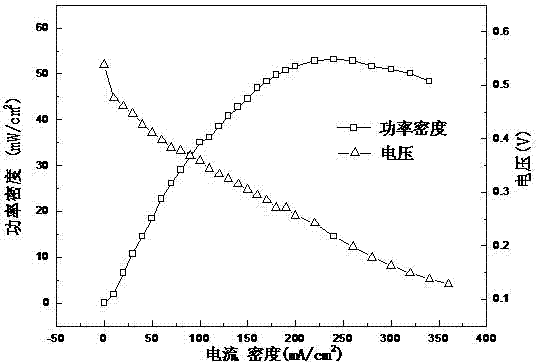
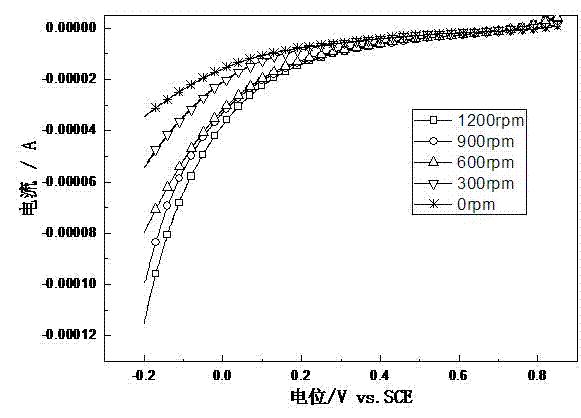
Image
Examples
Embodiment 1
[0035] (1) Mix 40 parts by weight of melamine-formaldehyde resin prepolymer with a solid content of 60% and a pH value of 7 to 9, and 10 parts by weight of pure water, and stir for 10 minutes to mix evenly;
[0036](2) Add 2 parts by weight of cobalt nitrate to the uniformly mixed melamine-formaldehyde resin prepolymer, and stir for 60 min to completely dissolve cobalt nitrate in the melamine-formaldehyde resin prepolymer;
[0037] (3) Add glacial acetic acid to the melamine-formaldehyde resin prepolymer dissolved in cobalt nitrate, adjust the pH value to 1.5, and let it stand for 10 minutes. After it is completely solidified, dry it under reduced pressure and vacuum for 3 hours at 30 °C to obtain a block Melamine formaldehyde xerogel containing cobalt nitrate;
[0038] (4) The massive cobalt nitrate-containing melamine-formaldehyde xerogel was heat-treated at 700°C for 1 h under the protection of Ar, cooled, ground into powder, and sieved with 300 mesh to obtain a spherical n...
Embodiment 2
[0041] (1) Mix melamine-formaldehyde resin prepolymer with a solid content of 60% in parts by weight of 40 parts and a pH value of 7 to 9 with purified water in parts by weight of 5 parts, and stir for 10 minutes to mix evenly;
[0042] (2) Add 2 parts by weight of cobalt acetate to the uniformly mixed melamine-formaldehyde resin prepolymer, and stir for 30 minutes to completely dissolve cobalt acetate in the melamine-formaldehyde resin prepolymer;
[0043] (3) Add 10% hydrochloric acid into the melamine-formaldehyde resin prepolymer dissolved with cobalt acetate, adjust the pH value to 2.0, let it stand for 30 minutes, and dry it under reduced pressure and vacuum for 3 hours at 30 °C after it is completely cured. Obtain block melamine formaldehyde xerogel containing cobalt acetate;
[0044] (4) The massive cobalt acetate-containing melamine formaldehyde xerogel was heat-treated at 750 °C for 1.0 h under the protection of Ar, cooled, ground into powder, and sieved with 300 mes...
Embodiment 3
[0047] (1) Mix 50 parts by weight of melamine-formaldehyde resin prepolymer with a solid content of 60% and a pH value of 7 to 9 with 10 parts of pure water and stir for 10 minutes to mix evenly;
[0048] (2) Add 5 parts by weight of iron acetate to the uniformly mixed melamine-formaldehyde resin prepolymer, and stir for 30 minutes to completely dissolve the iron acetate in the melamine-formaldehyde resin prepolymer;
[0049] (3) Add 15% nitric acid dropwise to the melamine-formaldehyde resin prepolymer dissolved in iron acetate, adjust the pH value to 3.0, let it stand for 5 h, and dry it under reduced pressure and vacuum for 3 h at 30 °C after it is completely cured , obtain blocky melamine formaldehyde xerogel containing iron acetate;
[0050] (4) The bulk melamine formaldehyde xerogel containing iron acetate was placed in N 2 Heat treatment at 700°C for 2 h under protection, grinding into powder after cooling, and sieving with 300 meshes to obtain a spherical nitrogen-dop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com