Application of phenol compounds in preparation of anti-complement medicines
A phenolic compound and compound technology are applied in the field of phenolic compounds and their new uses in the preparation of anti-complement drugs, and can solve the problems such as the anti-complement effect of phenolic compounds that have not been seen yet.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
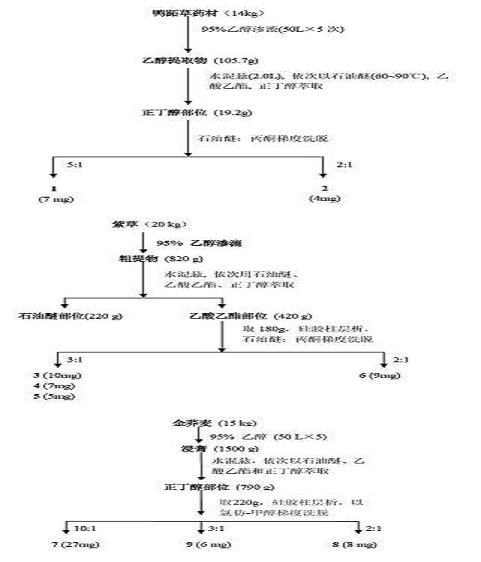
[0039] Embodiment 1 Preparation of phenolic compounds
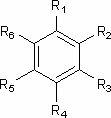
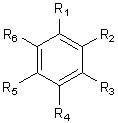
[0040]Take 14kg of Commelina chinensis, soak in ethanol at room temperature (50L×5 times), combine the extracts and concentrate until there is no alcohol smell, dilute the extracts with water to 2L, and extract with petroleum ether, ethyl acetate, and n-butanol in sequence ( Each 2L x 3 times), the combined n-butanol extracts were concentrated to dryness to obtain 19.2g of n-butanol extracts; , acetone gradient elution, and the fractions were subjected to repeated column chromatography, SephadexLH-20 purification and preparative chromatography to obtain phenolic compounds ( 1 )and( 2 ),Specific steps are as follows:
[0041] ① The obtained fractions were eluted with petroleum ether-acetone (5:1). After repeated column chromatography, using chloroform-methanol as eluent, gradient elution, the fractions were purified by SephadexLH-20 and eluted with methanol to obtain the compound ( 1 ) (7 mg). Using spectroscopic anal...
Embodiment 2
[0043] The preparation of embodiment 2 phenolic compounds
[0044] Take 20kg of dried roots of Comfrey, and the coarse powder is cold soaked and percolated several times with 95% ethanol at room temperature, and then the solvent is recovered under reduced pressure to obtain 820g of extract, which is suspended in distilled water and mixed with petroleum ether and ethyl acetate. Ethyl acetate and n-butanol were extracted to obtain 420 g of ethyl acetate extract; 180 g of ethyl acetate extract was subjected to silica gel column chromatography, and petroleum ether (60-90°C), petroleum ether (60-90°C)-acetone, acetone gradient Elution, the resulting fraction was subjected to repeated silica gel column chromatography with different eluents, purified by SephadexLH-20 and separated by preparative chromatography to obtain the compound ( 3 ), ( 4 ), ( 5 )and( 6 ),Specific steps are as follows:
[0045] ① The fractions eluted with petroleum ether-acetone (3:1) were subjected to sili...
Embodiment 3
[0048] Example 3 Preparation of phenolic compounds
[0049] Take 15kg of golden buckwheat rhizome powder, cold-soak with ethanol at room temperature (50L×5 times), combine the extracts and concentrate until there is no alcohol smell, add water to dilute the extracts to 2L, and extract with petroleum ether, ethyl acetate, and n-butanol in sequence ( 2L × 4 times each), the combined n-butanol extracts were concentrated to dryness to obtain 790 g of n-butanol extracts; 220 g of n-butanol fractions were dry-loaded for silica gel column chromatography, and chloroform, chloroform-methanol , methanol gradient elution, the obtained fractions were subjected to repeated silica gel column chromatography with different eluents, SephadexLH-20 purification and preparative chromatography, and the compound ( 7 ), ( 8 )and( 9 ).
[0050] ① The obtained fractions were eluted with chloroform-methanol (10:1), subjected to silica gel column chromatography, petroleum ether-acetone (4:1) was used...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com