Benzimidazolyl thiazolidinone compounds and synthesis method thereof
A benzimidazole-based, thiazolidinedione technology, applied in the direction of organic chemistry, drug combination, metabolic diseases, etc., can solve the problems of low fat solubility, difficult to penetrate the blood-brain barrier, unable to reach diabetic encephalopathy, etc., and achieve fat solubility. Strong, easy to cross the blood-brain barrier effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
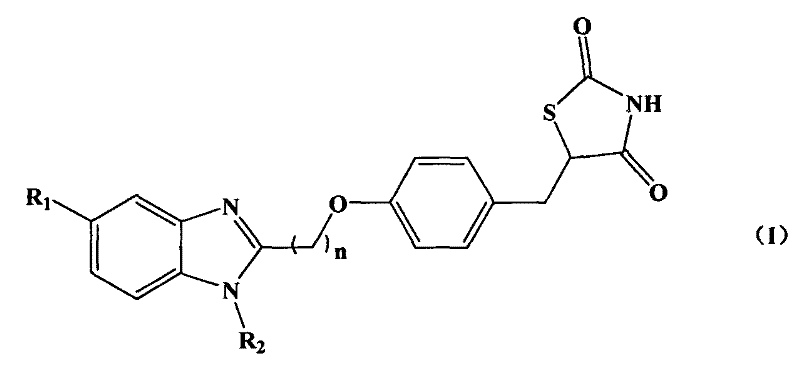
[0049] Example 1: Synthesis of 5-((4-((1H-benzoimidazolyl)methoxy)phenyl)methyl)thiazole-2,4-dione (HJ-2)
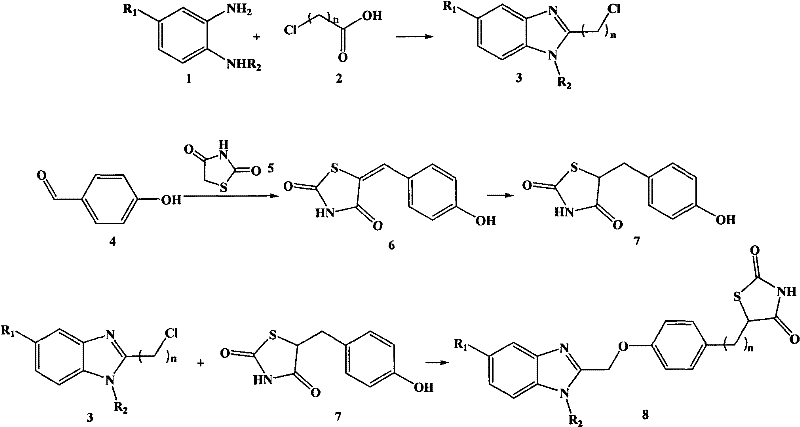
[0050] (1) The synthesis of compound 2-chloromethyl-1H-benzimidazole, the synthetic steps are as follows:
[0051]
[0052] Add 0.54g (5.0mmol) of o-phenylenediamine, 0.567g (6.0mmol) of chloroacetic acid and 30mL of 1mol / L hydrochloric acid into a 50mL three-neck flask equipped with a reflux condenser, stir with a magnet, raise the temperature to 90°C for 12h, and cool After reaching room temperature, the reaction solution was poured into a mixed solution of ice and water, and the pH was adjusted to 8 with 1 mol / L sodium hydroxide, and a flesh-colored powder precipitated out. Suction filtration and drying yielded 0.76 g of crude product with a yield of 91.30%.
[0053] Mass spectrum MS, ESI-: 166.82 (M-H), (calcd.166.61);
[0054] Nuclear magnetic 1H NMR (DMSO) δ (ppm) 4.92 (s, 2H), 7.20-7.22 (m, 2H), 7.55-7.57 (m, 2H).
[0055] (2) Synthesis of compound 5-(4-hydr...
Embodiment 2
[0070] Example 2: Synthesis of 5-((4-((5-methyl-1H-benzoimidazolyl)methoxy)phenyl)methyl)thiazole-2,4-dione (HJ-4)
[0071] (1) The synthesis of compound 2-(chloromethyl)-5-methyl-1H-benzimidazole is similar to Example 1 step (1), except that 3-methyl o-phenylenediamine replaces o-phenylenediamine , yield 70.00%.
[0072] (2) For the synthesis of compound 5-(4-hydroxybenzylidene)thiazole-2,4-dione, see step (2) of Example 1.
[0073] (3) Synthesis of compound 5-(4-hydroxybenzyl)thiazole-2,4-dione See step (3) of Example 1.
[0074] (4) 5-((4-((5-methyl-1H-benzimidazolyl)methoxy)phenyl)methyl)thiazole-2,4-dione (HJ-4) and Example 1 step
[0075] (4) similar, yield 80.30%.
Embodiment 3
[0076] Example 3: Synthesis of 5-((4-((1-methyl-1H-benzoimidazolyl)methoxy)phenyl)methyl)thiazole-2,4-dione (HJ-6)
[0077] (1) The synthesis of compound 2-(chloromethyl)-5-methyl-1H-benzimidazole is similar to Example 1 step (1), except that N-methyl o-phenylenediamine replaces o-phenylenediamine , yield 70.00%.
[0078] (2) For the synthesis of compound 5-(4-hydroxybenzylidene)thiazole-2,4-dione, see step (2) of Example 1.
[0079] (3) Synthesis of compound 5-(4-hydroxybenzyl)thiazole-2,4-dione See step (3) of Example 1.
[0080] (4) 5-((4-((1-methyl-1H-benzimidazolyl)methoxy)phenyl)methyl)thiazole-2,4-dione (HJ-6) and Example 1 Step (4) is similar, yield 90.00%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com