A cooling phase change material with a phase change temperature of 12-29°C and its preparation method
A phase-change material and phase-change temperature technology, applied in the field of material chemistry, can solve the problems of inability to achieve cooling effect, weak feeling of coldness, insufficient phase separation, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Mix and stir 1g of glycerin and 1g of borax, add 1g of boric acid to adjust the pH value to 6, add 0.5g of sodium chloride, 0.5g of ammonium chloride and 1g of sodium polyacrylate and stir, mix well and then add 95g of sodium sulfate decahydrate, Stir at 29°C until melting, stop heating, continue stirring, the temperature of the mixture decreases, and the flocculent solid obtained after cooling is the phase change material of the present invention.
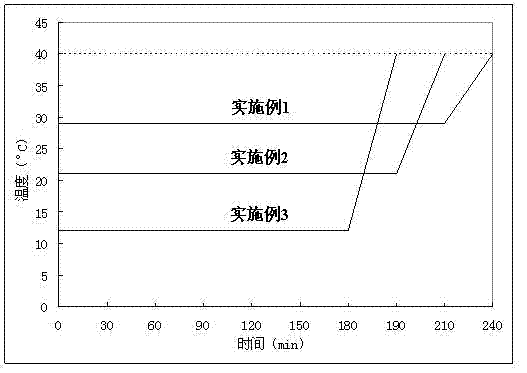
[0026] Depend on figure 1 It can be seen that the phase change temperature of the phase change material prepared in Example 1 is 29° C., and the latent heat of phase change is 190 J / g.
Embodiment 2
[0028] 3g glycerol and 3g borax were mixed and stirred, 3g boric acid was added to adjust the pH value to 7.5, 3.5g sodium chloride, 3.5g potassium chloride, 3.5g ammonium chloride, 3.5g ammonium sulfate and 2g polyacrylamide were added thereto and stirred, After mixing evenly, add 75g of sodium sulfate decahydrate, stir at 25°C until melting, stop heating, continue stirring, the temperature of the mixture decreases, and the flocculent solid obtained after cooling is the phase change material of the present invention.
[0029] Depend on figure 1 It can be seen that the phase change temperature of the phase change material prepared in Example 2 is 21° C., and the latent heat of phase change is 165 J / g.
Embodiment 3
[0031] Mix and stir 10g of glycerin and 5g of borax, add 5g of boric acid to adjust the pH value to 8, add 12.5g of potassium chloride, 12.5g of ammonium sulfate and 5g of sodium carboxymethylcellulose and stir, then add 50g of sulfuric acid decahydrate after mixing evenly Stir the sodium at 12°C until it melts, stop heating, continue stirring, the temperature of the mixture decreases, and the flocculent solid obtained after cooling is the phase change material of the present invention.
[0032] Depend on figure 1 It can be seen that the phase change temperature of the phase change material prepared in Example 3 is 12° C., and the latent heat of phase change is 140 J / g.
[0033] The phase-change material of the present invention adopts a phase-change temperature regulator. Within a certain range, with the increase of the temperature regulator, the phase-change temperature of the material gradually decreases, so that the phase-change temperature can be adjusted between 12-29°C....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Phase transition temperature | aaaaa | aaaaa |
| Latent heat of phase change | aaaaa | aaaaa |
| Phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com