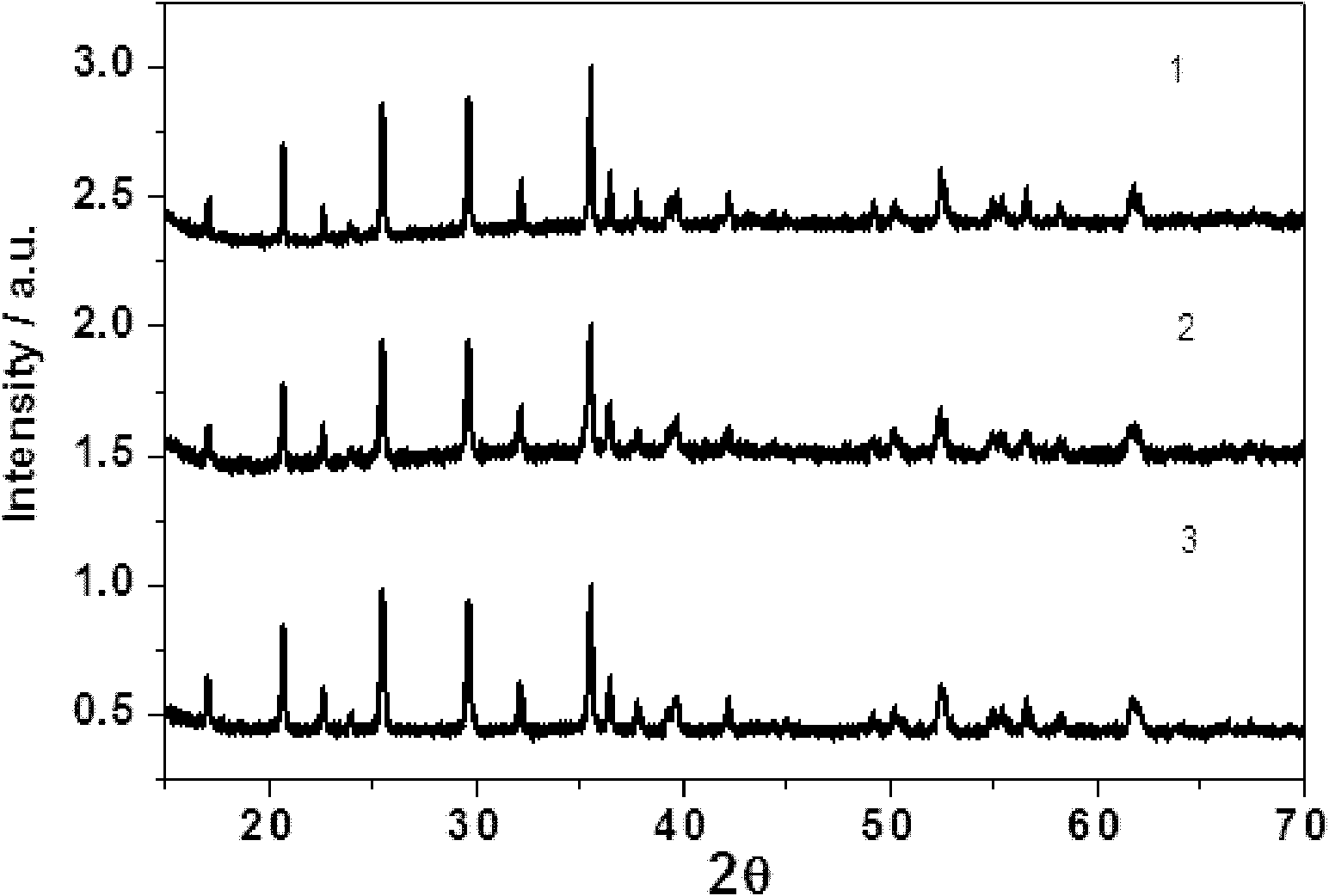
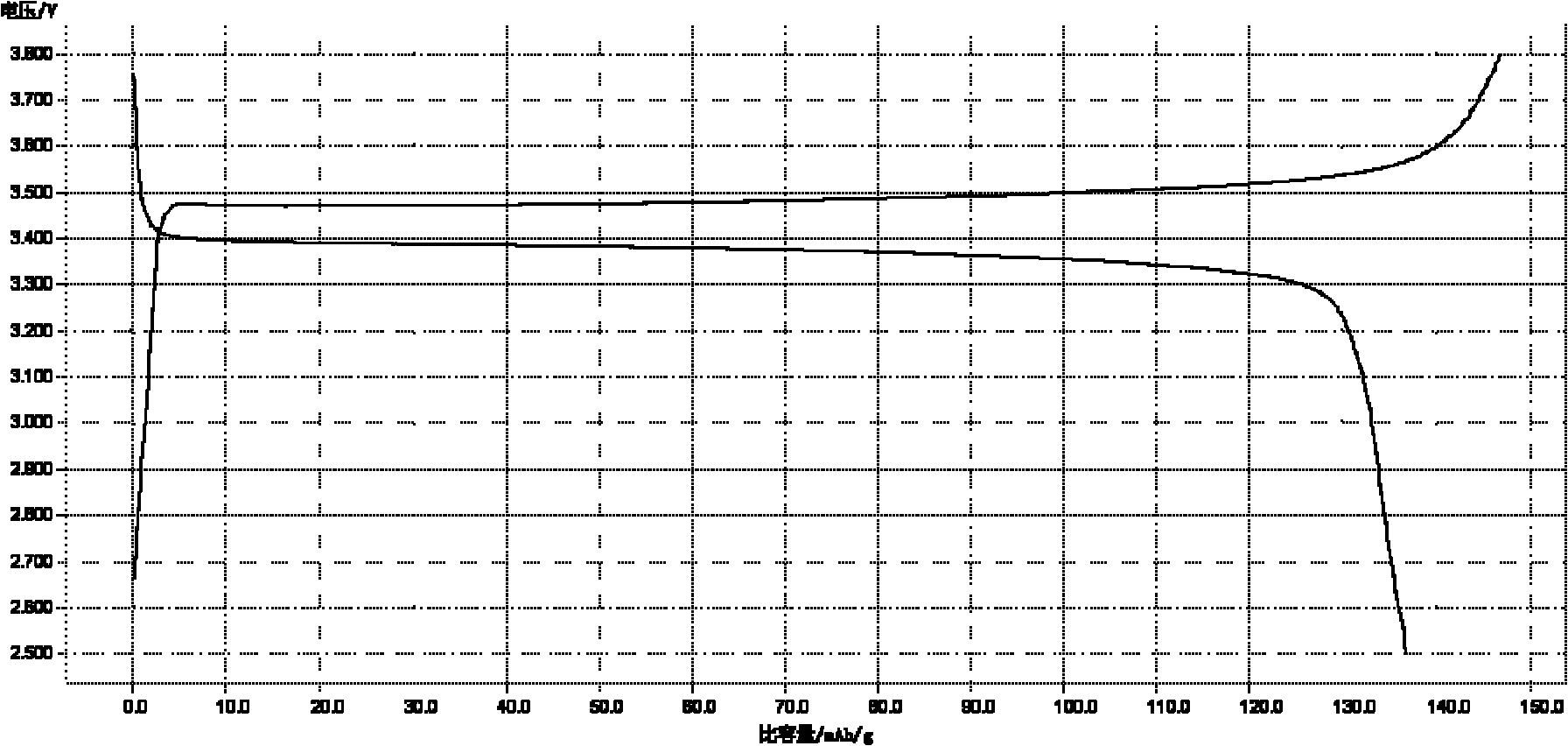
A kind of microwave synthesis method of nano-lithium iron phosphate
A lithium iron phosphate, microwave synthesis technology, applied in the direction of phosphorus compounds, nanotechnology, chemical instruments and methods, etc., can solve the problems of limited phase purity, large power consumption, high cost, etc., to shorten the production cycle, save energy, particle uniform effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] aWeigh 1molFeSO according to the molar ratio of 1:1 4 , 1molH 3 PO 4 (calculated by the effective content of phosphoric acid at 85% concentration), be dissolved in 2000ml of deionized water, then add 0.1mol complexing agent ethylene glycol, under constant stirring, slowly add 3000ml of 3mol lithium hydroxide solution to form mixed solution A;
[0021] b. Continuously stir the mixed solution A obtained in step a in an oil bath at 110° C. for 1 h until a green precipitate is produced, and the green precipitate is suction-filtered and washed to obtain a solid product;
[0022] c. Dry the solid product obtained in step b at 40° C. for 2 hours in a microwave vacuum dryer to obtain a precursor, and then perform ball milling on the precursor to obtain a spare precursor powder with a particle size of 20 nm;
[0023] d Put the standby precursor powder and high molecular polymer solution obtained in step c into a closed vacuum container, and then add a heat conducting agent, wh...
Embodiment 2
[0026] aWeigh 1molFeSO according to the molar ratio of 1:1 4 , 1molH 3 PO 4 (according to the effective content calculation of 85% concentration phosphoric acid), be dissolved in the deionized water of 2000ml, then add 0.1mol complexing agent triethanolamine, under constantly stirring, slowly add the lithium hydroxide solution 3000ml of 3mol, form mixed solution A;
[0027] b. The mixed solution A obtained in step a was continuously stirred in an oil bath at 130°C for 1.5h until a green precipitate was produced, and the green precipitate was subjected to suction filtration and washed to obtain a solid product;
[0028] c. Dry the solid product obtained in step b in a microwave vacuum dryer at 50° C. for 1.5 h to obtain a precursor, and then perform ball milling on the precursor to obtain a spare precursor powder with a particle size of 35 nm;
[0029] d Put the standby precursor powder and high molecular polymer solution obtained in step c into a closed vacuum container, and...
Embodiment 3
[0032] aWeigh 1molFeSO according to the molar ratio of 1:1 4 , 1molH 3 PO 4 (according to the effective content calculation of 85% concentration phosphoric acid), be dissolved in the deionized water of 2000ml, then add 0.1mol complexing agent polypropylene, under constantly stirring, slowly add the lithium hydroxide solution 3000ml of 3mol, form mixed solution A;
[0033] b. The mixed solution A obtained in step a was continuously stirred in an oil bath at 140°C for 2 hours until a green precipitate was produced, and the green precipitate was subjected to suction filtration and washed to obtain a solid product;
[0034] c. Drying the solid product obtained in step b at 60° C. for 1 h in a microwave vacuum dryer to obtain a precursor, and then performing ball milling on the precursor to obtain a spare precursor powder with a particle size of 50 nm;
[0035]d Put the standby precursor powder and high molecular polymer solution obtained in step c into a closed vacuum container,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com