Preparation methods of losartan potassium and preparation thereof
A technology of losartan potassium and losartan, applied in the field of chemical pharmacy, can solve problems such as high production cost and complicated subsequent procedures, and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0012] Below, in conjunction with embodiment the content of the present invention is described in detail.
[0013] 1, the preparation of raw material losartan potassium
[0014] Detailed steps
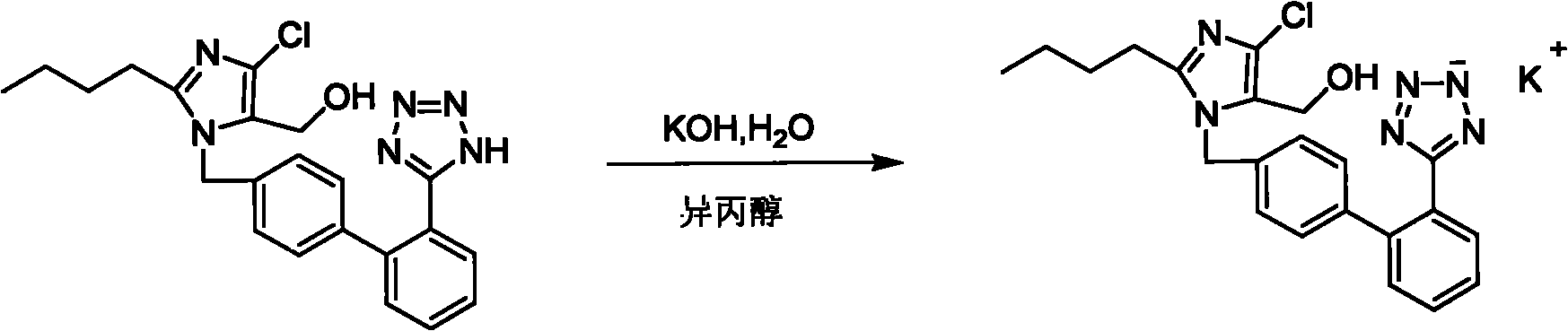
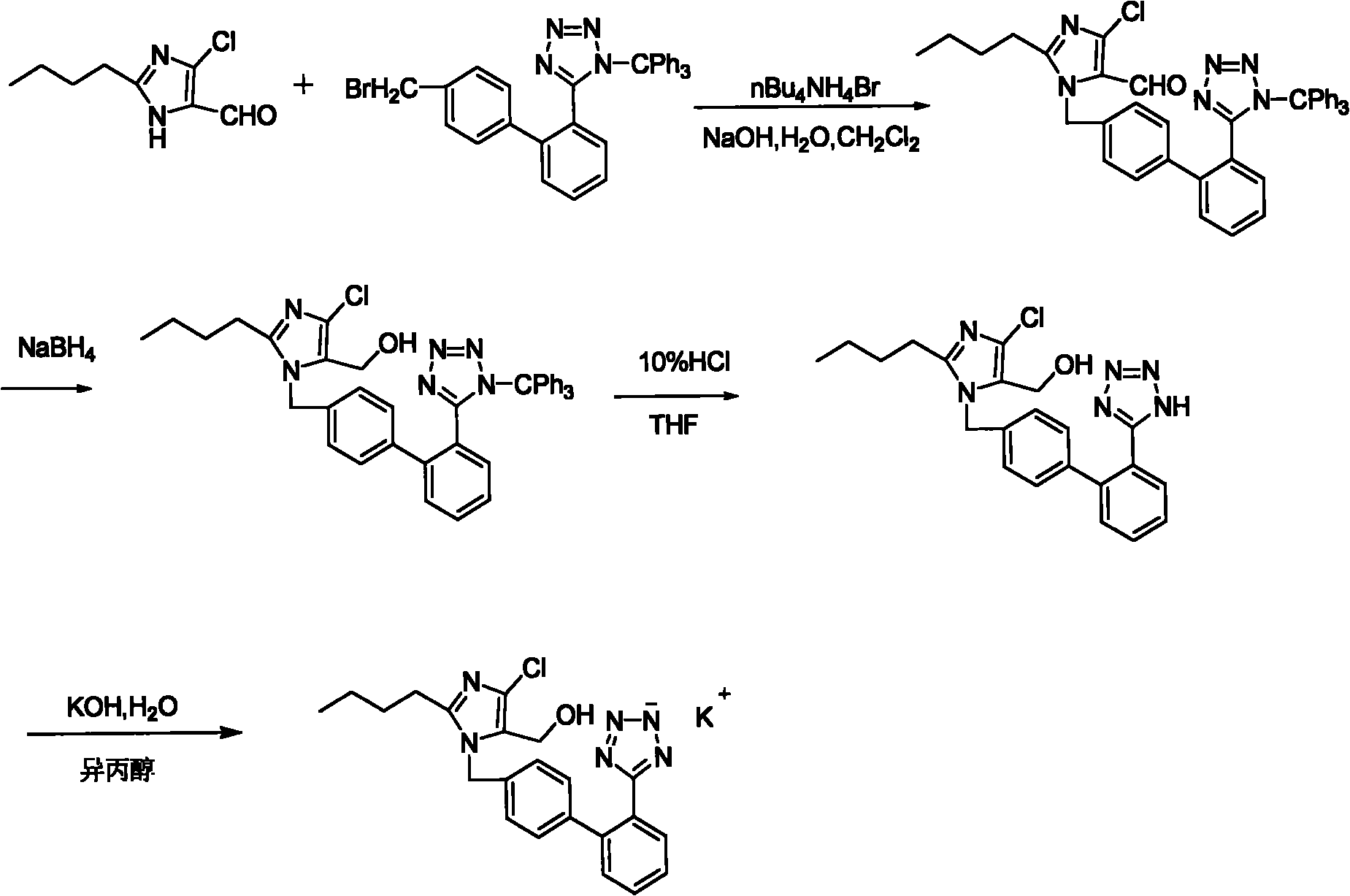
[0015] ①2-Butyl-4-chloro-5-(hydroxymethyl)-1-[[2'-[(triphenylmethyl)-tetrazolium-5-]biphenyl-4-]methyl] Imidazole (trityl losartan)
[0016] Main feeding ratio:
[0017] 2-Butyl-5(4)-formyl 186.5g (FW: 186.5) 1.0mol-4(5)-chloroimidazole(II)
[0018] N-(triphenylmethyl)-5-[(4'-557.0g (FW:557) 1.0mol bromomethyl)-biphenyl-2-]tetraazol
[0019] Azole(III)
[0020] Tetrabutylammonium bromide 32.2g (FW: 322) 0.1mol
[0021] Sodium hydroxide 80.0g (FW: 40) 2.0mol
[0022] Sodium borohydride 38.0g (FW: 38) 1.0mol
[0023] 2-Butyl-5(4)-formyl-4(5)-chloroimidazole (II) 186.5g (1.0mol), tetrabutylammonium bromide 32.2g (0.1mol), 1.0mol / L hydroxide Mix 2000ml (2.0mol) of sodium with 2.0L of dichloromethane, and add N-(triphenylmethyl)-5-[(4'-bromomethyl)-biphenyl-2-]tetrazolium ( III) ...
PUM
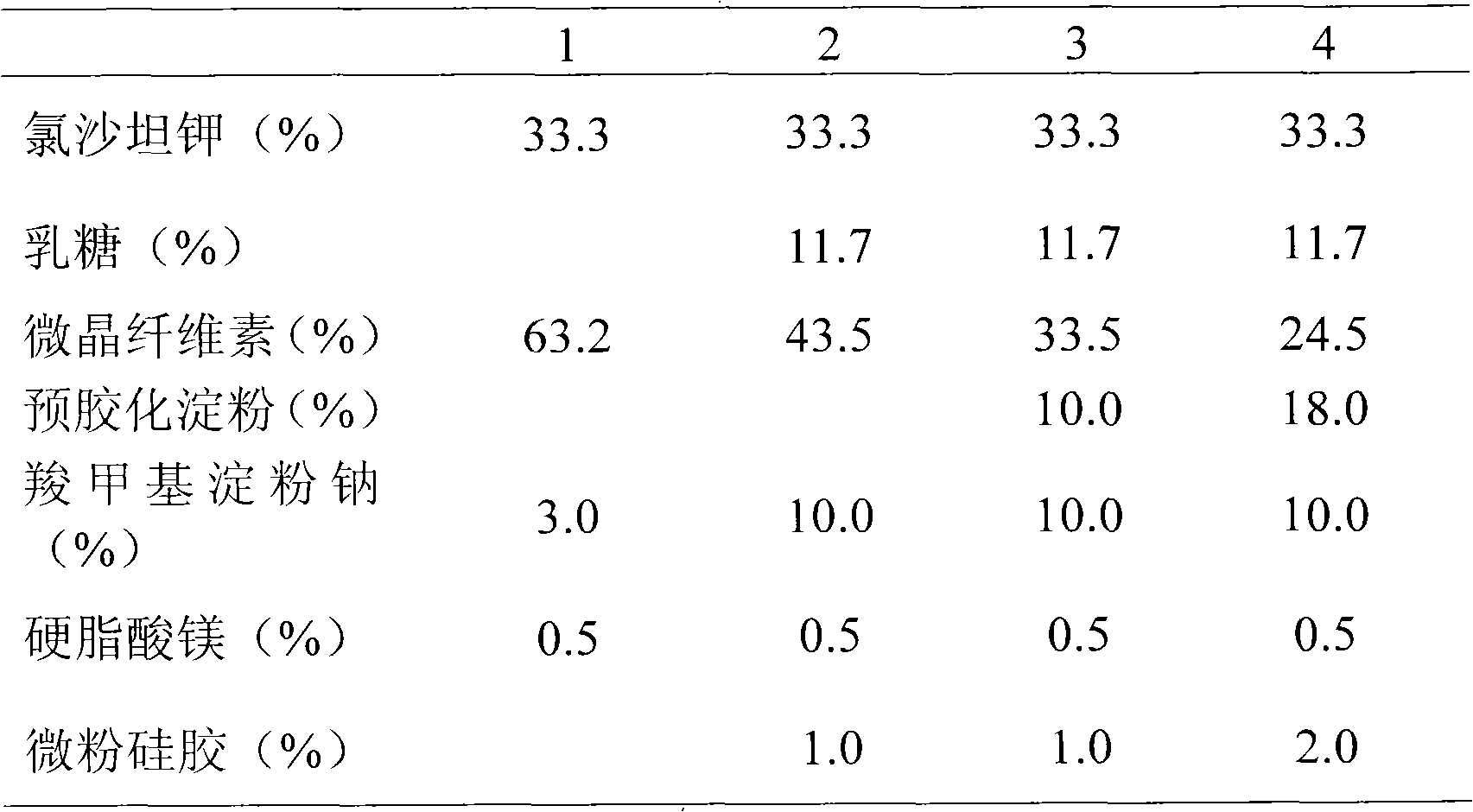
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com