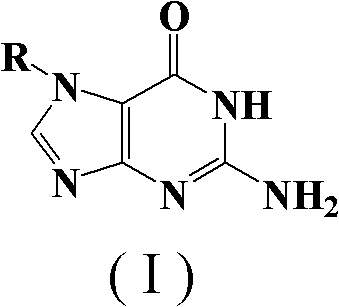
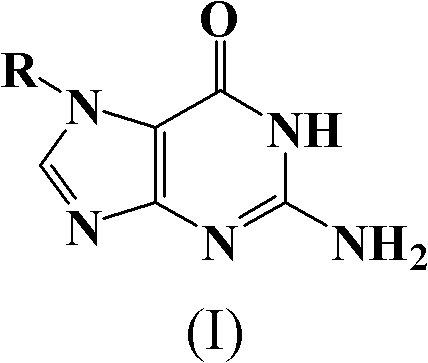
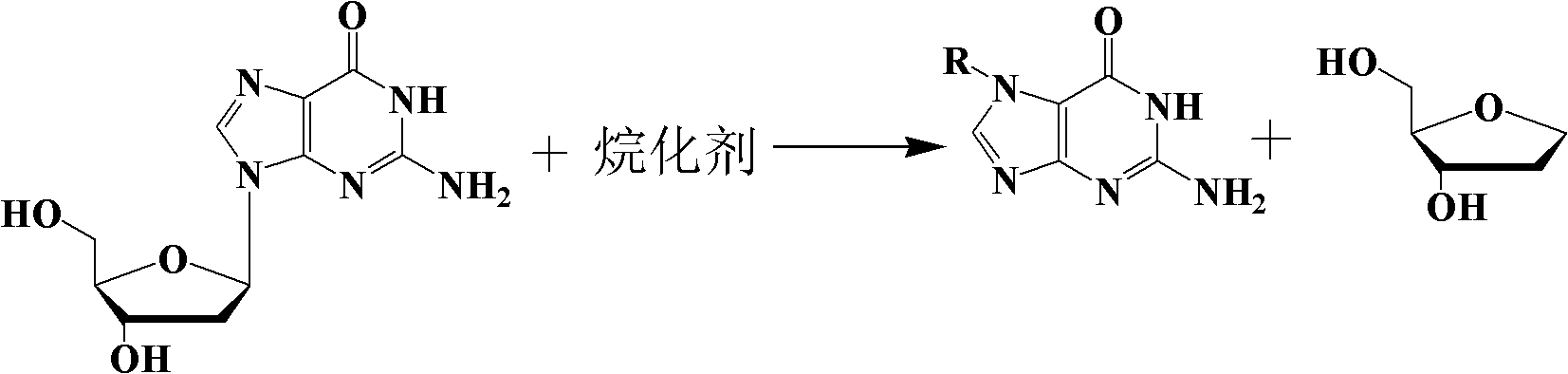
The preparation method of n7-guanine alkylate
An alkylate and guanine technology, which is applied in the field of preparation of N7-guanine alkylate, can solve the problems of cumbersome preparation process, complicated separation and purification, poor applicability and the like, and achieves simple separation process, good solubility, and economical washing. The effect of de-agent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 N 7 - Preparation of ethylguanine
[0032]
[0033] Add 300mg (1.12mmol) of 2'-deoxyguanosine and 6mL of dimethylacetamide into a 25mL round-bottomed flask, and sonicate for 1min. After the 2'-deoxyguanosine is completely dissolved, add iodoethane 0.6mL (7.42 mmol), put into a stirring bar, airtightly stir, react at 25 DEG C for 24h, after the completion of the reaction, distill off the solvent under reduced pressure at 23 DEG C, purify through silica gel column chromatography, use ethyl acetate:petroleum ether (volume ratio)=12 : 1 eluent elution, and then ethyl acetate: anhydrous methanol (volume ratio) = 12: 1 eluent elution to obtain the final product N 7 -Ethylguanine 189mg, the product is a yellow solid, and the yield is 95.3%.
[0034] UVλ: 245, 284nm; 1 H NMR (400MHz, DMSO-d 6 ): δ1.78~1.74 (m, 3H, CH 3 ), 3.87~3.84 (m, 2H, CH 2 ), 6.16(s, 2H, NH 2 ), 7.82 (s, 1H, CH), 11.00 (s, 1H, NH); MS (ESI): m / z 180 (M+H + ).
Embodiment 2
[0035] Example 2 N 7 - Preparation of ethylguanine
[0036]Add 360mg (1.35mmol) of 2'-deoxyguanosine and 9mL of dimethylacetamide into a 25mL round-bottomed flask, and sonicate for 1.5min. After the 2'-deoxyguanosine is completely dissolved, add 1mL of iodoethane (12.37 mmol), put into a stirring bar, airtightly stir, react at 25 DEG C for 20h, after the completion of the reaction, distill off the solvent under reduced pressure at 25 DEG C, purify through silica gel column chromatography, use ethyl acetate:petroleum ether (volume ratio)=15 : 1 eluent elution, and then ethyl acetate: anhydrous methanol (volume ratio) = 15: 1 eluent elution to obtain the final product N 7 -Ethylguanine 234mg, the product is a yellow solid, and the yield is 97.5%. The UV, NMR and mass spectrum data of the product are the same as in Example 1.
Embodiment 3
[0037] Example 3 N 7 Preparation of -(2-hydroxyethyl)guanine
[0038]
[0039] Add 480mg (1.80mmol) of 2'-deoxyguanosine and 9mL of glacial acetic acid into a 25mL round-bottomed flask, sonicate for 2min, and then add 0.6mL (11.86mmol) of ethylene oxide after the 2'-deoxyguanosine is completely dissolved , put into a stirring bar, airtightly stir, and react at 37°C for 1h, after the reaction was completed, the solvent was distilled off under reduced pressure at 35°C, and purified by silica gel column chromatography, using ethyl acetate:petroleum ether (volume ratio)=15:1 The eluent was eluted, and then eluted with ethyl acetate: anhydrous methanol (volume ratio) = 12: 1 eluent to obtain the final product N 7 -Hydroxyethylguanine 339mg, the product is a white solid, and the yield is 96.6%.
[0040] UVλ: 247, 284nm; 1 H NMR (400MHz, DMSO-d 6 ): δ3.73~3.69 (m, 2H, CH 2 ), 4.14~4.12 (m, 2H, CH 2 ), 6.39 (s, 2H, NH 2 ), 8.08 (s, 1H, CH), 10.67 (s, 1H, NH); MS (ESI): m / z 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com