Preparation method of perfluorooctanoic acid artificial complete antigen
A technology of perfluorooctanoic acid and complete antigen, applied in chemical instruments and methods, animal/human proteins, instruments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
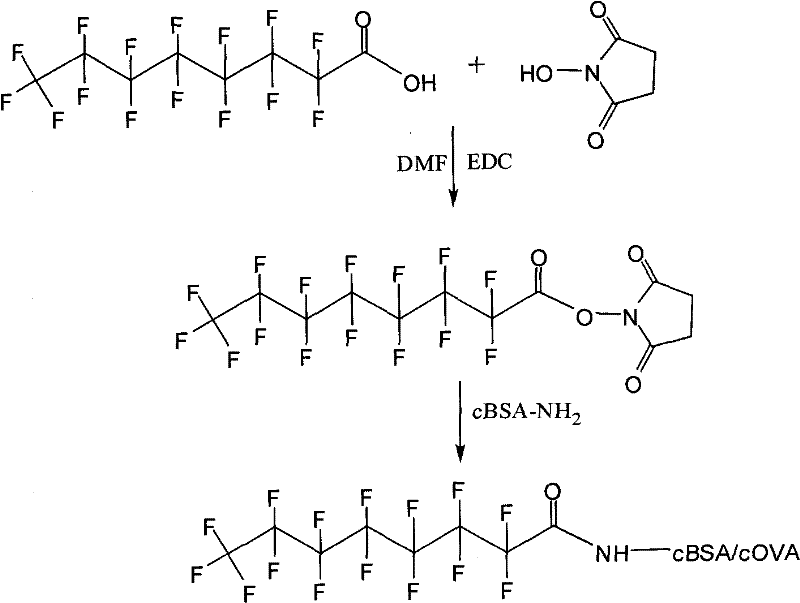
[0006]
[0007] Perfluorooctanoic acid (10mg), NHS (14mg), EDC (40mg) were dissolved in DMF (3ml), under nitrogen protection, reacted at room temperature for 10 hours; then the above reaction mixture was centrifuged, and the supernatant was slowly added dropwise to BSA ( 40mg) in PBS buffer solution (20ml). React at room temperature for 5 hours, then centrifuge, put the supernatant into a dialysis bag prepared by a dialysis membrane with a molecular weight of 10,000, suspend the dialysis bag in PBS buffer solution in a 2000ml beaker, dialyze at 4°C for 7 days, and then put the dialysis bag The liquid freeze-dried in is the complete antigen of synthetic perfluorooctanoic acid. The molecular weight of the antigen determined by MALDI-TOF-MS mass spectrometry is: 72593.
Embodiment 2
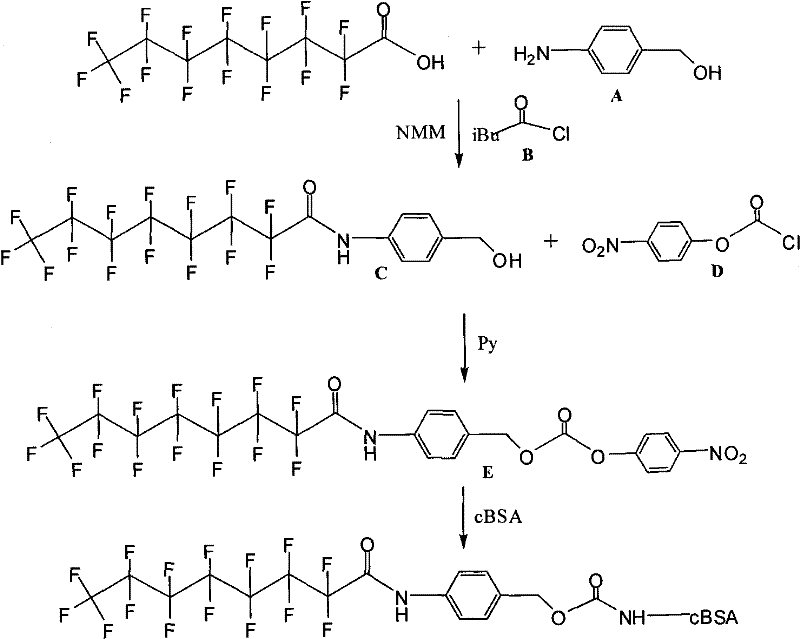
[0009]
[0010] Dissolve perfluorooctanoic acid (100mg), compound A (40mg), and compound B (0.05ml) in NMM (3ml), under nitrogen protection, react at -20°C for 5 hours, perform conventional organic reaction treatment, and obtain compound C after column chromatography Pure. Compound C (200 mg) and compound D (230 mg) were dissolved in pyridine, DMAP (250 mg) was added at 0°C, reacted for 5 hours, conventional organic reaction treatment, and purified compound E was obtained after column chromatography. Compound E (40 mg) was dissolved in DMF (2 ml), and then slowly added dropwise to a PBS buffer solution (20 ml) of BSA (140 mg). React at room temperature for 5 hours, then centrifuge, put the supernatant into a dialysis bag prepared by a dialysis membrane with a molecular weight of 10,000, suspend the dialysis bag in PBS buffer solution in a 2000ml beaker, dialyze at 4°C for 7 days, and then put the dialysis bag The liquid freeze-dried in is the complete antigen of synthetic ...
Embodiment 3
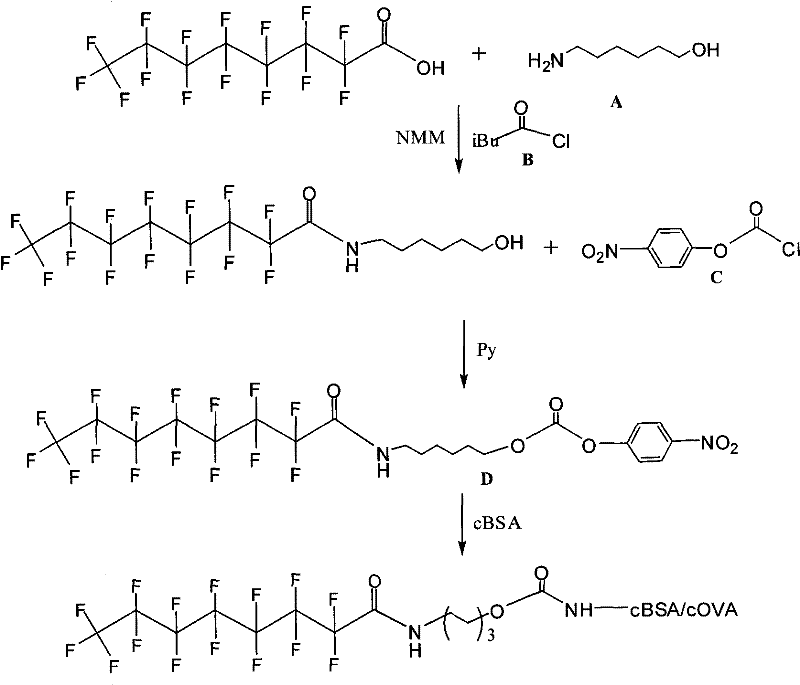
[0012]
[0013] Dissolve perfluorooctanoic acid (100mg), compound A (40mg), and compound B (0.05ml) in NMM (3ml), under nitrogen protection, react at -20°C for 5 hours, perform conventional organic reaction treatment, and obtain compound C after column chromatography Pure. Compound C (200 mg) and compound D (230 mg) were dissolved in DCM, DMAP (250 mg) was added at 0°C, reacted for 5 hours, conventional organic reaction treatment, and purified compound E was obtained after column chromatography. Compound E (40 mg) was dissolved in DMF (2 ml), and then slowly added dropwise to a PBS buffer solution (20 ml) of BSA (140 mg). React at room temperature for 5 hours, then centrifuge, put the supernatant into a dialysis bag prepared by a dialysis membrane with a molecular weight of 10,000, suspend the dialysis bag in PBS buffer solution in a 2000ml beaker, dialyze at 4°C for 7 days, and then put the dialysis bag The liquid freeze-dried in is the complete antigen of synthetic perfl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com