A kind of doripenem hydrate crystal and preparation method thereof
A technology of doripenem and hydrate, which is applied in the field of doripenem hydrate crystals and its preparation, can solve the problems that the crystal form IV cannot be directly obtained, the preparation process is complicated, and the preparation cost is high, so as to achieve good stability and high production cost. Simple, pure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Dissolve 5g of crude doripenem in 100mL of distilled water at 50-55°C, then cool down to 25°C in a water bath, add 0.5g of activated carbon, stir for 15 minutes to decolorize; Precipitation, stirring for two hours, suction filtration, washing the filter cake with isopropanol / water = 4:1; drying at 50-60° C., 5 mmHg for 1.5 hours, to obtain 2 g of doripenem hydrate crystals of the present invention.
[0053] Refer to the method described in "Journal of Chromatography B, 853 (2007), 123-126" to detect the HPLC purity of the obtained crystals; Residual amount of isopropanol in the crystals.
[0054] It is found through detection that the HPLC purity of the crystals obtained in this example is 99.85%, and the residual amount of isopropanol is 219ppm.
[0055] The water content in the crystal was determined to be 4.83% by Karl Fischer (KF) method.
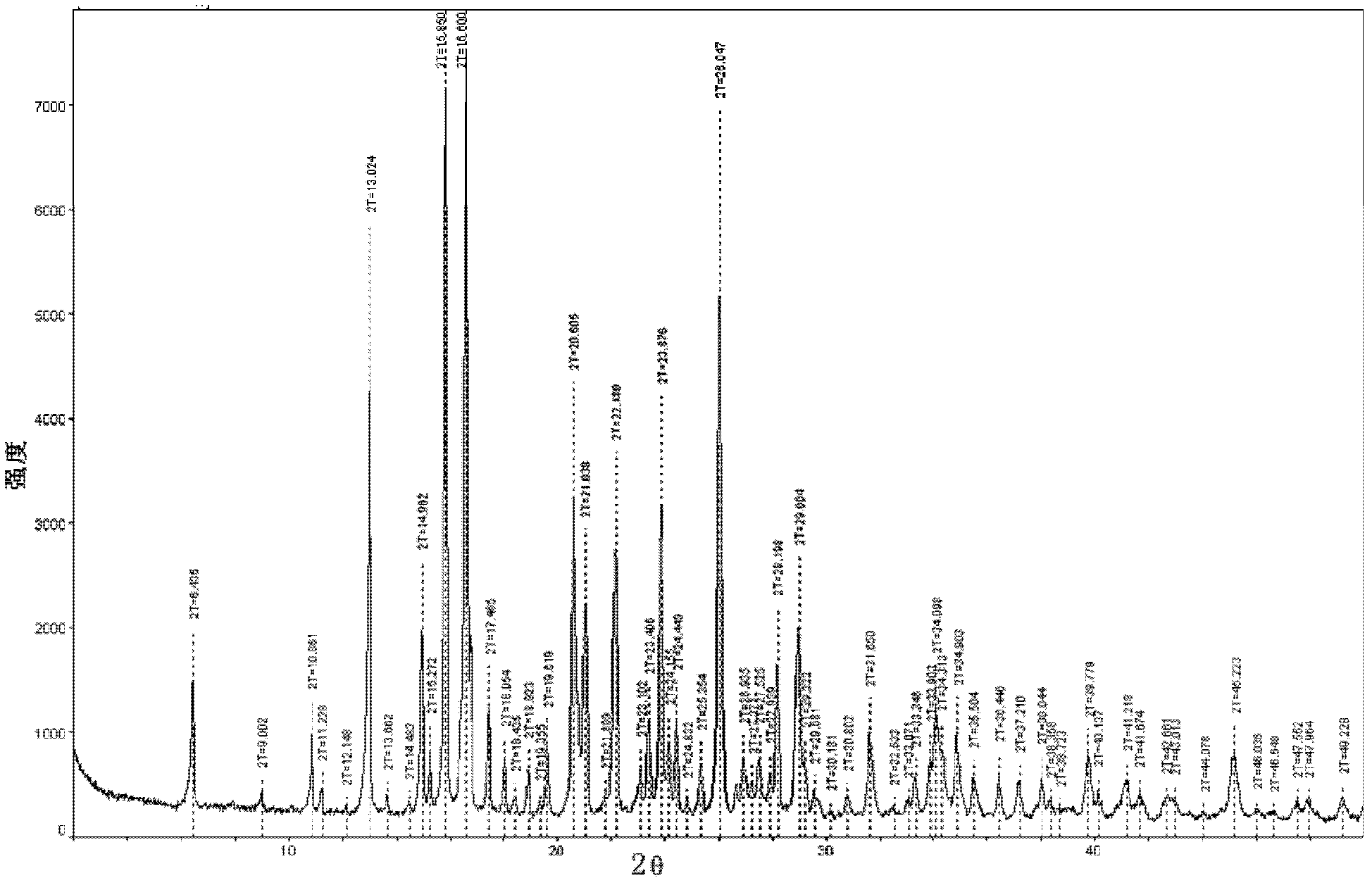
[0056] The powder X-ray diffraction spectrogram of the crystal obtained in the present embodiment is as follows figure 1 Sho...
Embodiment 2
[0061] Dissolve 10g of crude doripenem in 200mL of distilled water at 50-55°C, then cool down to 25°C in a water bath, add 1.0g of activated carbon, stir for 15 minutes to decolorize; filter with suction, cool the filtrate to about 0-10°C, and add The 0.1g crystal obtained in Example 1 is used as a seed crystal, and solids are precipitated. After stirring for 1 hour, 100ml of isopropanol is added dropwise, and after the dripping is completed and stirred for 2 hours, suction filtration is performed, and the filter cake is washed with isopropanol / water=4:1; Dry at 50-60°C and 10mmHg for 2 hours to obtain 8.2g of crystals.
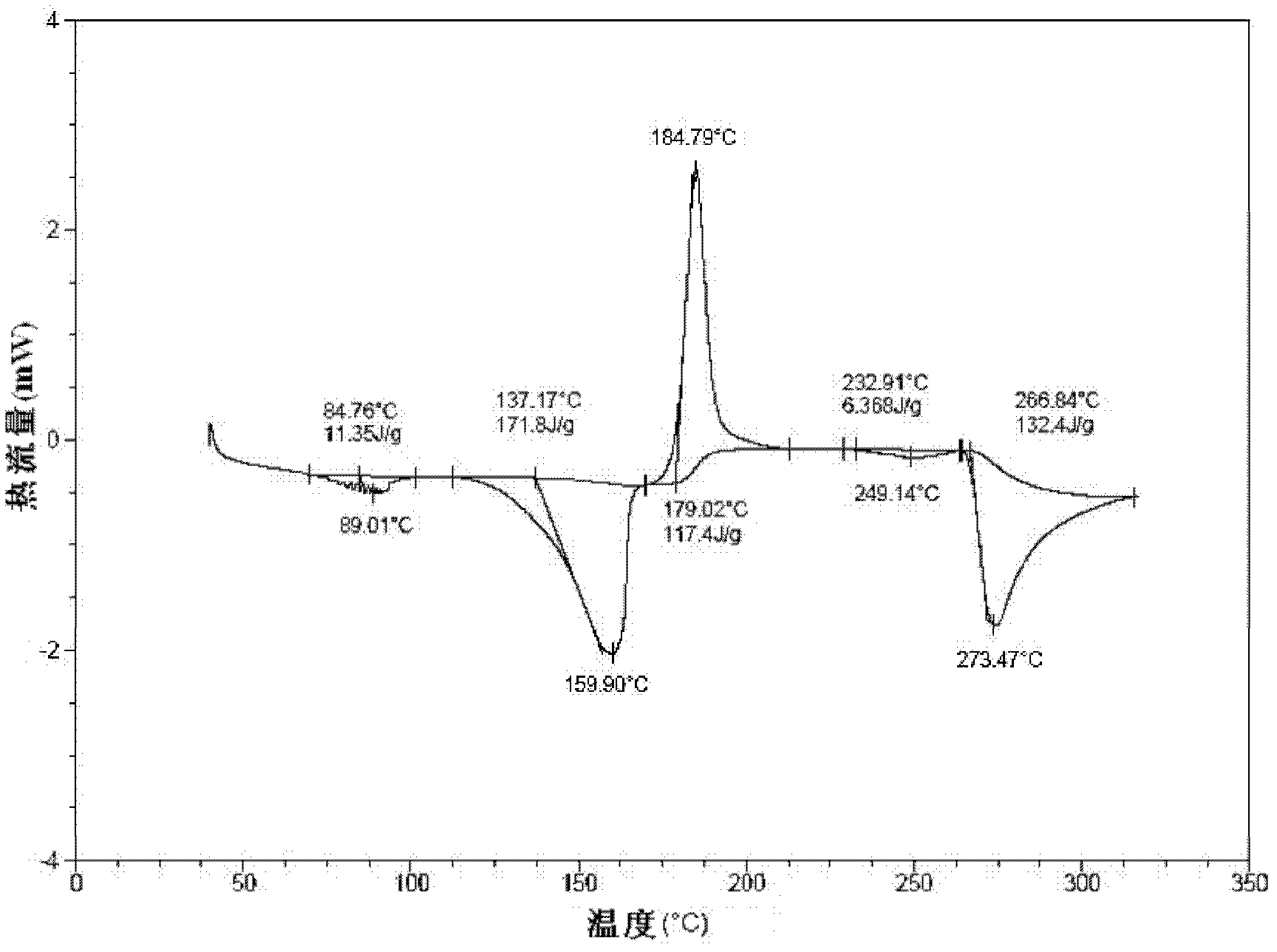
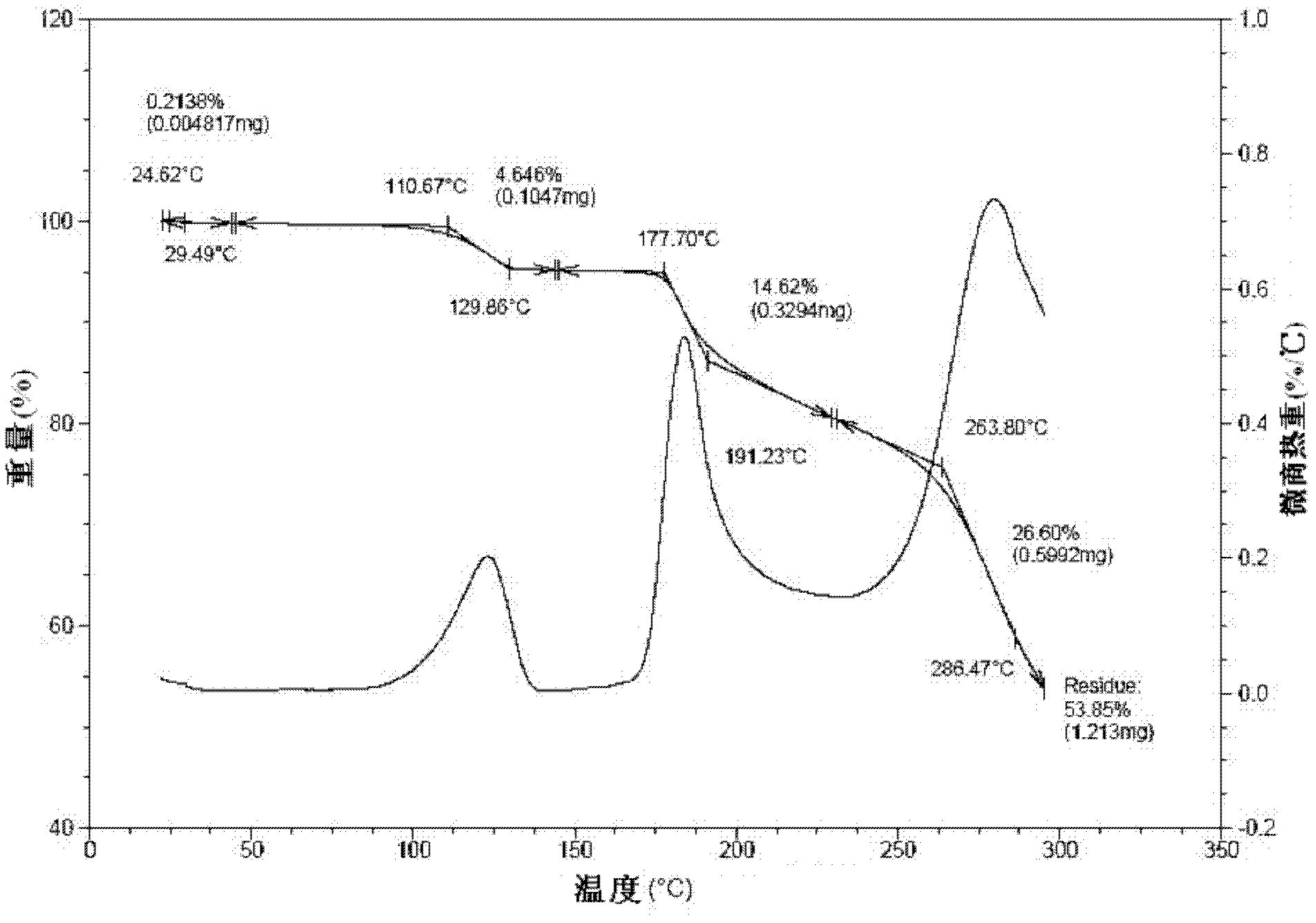
[0062] It is detected that: the HPLC purity of the crystal obtained in this embodiment is 99.86%, the residual amount of isopropanol in it is 205ppm, and the moisture measured by the Karl-Fischer (KF) method is 4.79%; and has figure 1 The powder X-ray diffraction spectrum shown, figure 2 The DSC spectrum shown, image 3 The TG spectrum shown and Figure 4...
Embodiment 3
[0064] Dissolve 10 g of crude doripenem in 110 mL of distilled water at 55-60 °C, then cool down to 15 °C in a water bath, add 1.0 g of activated carbon, stir for 15 minutes to decolorize; filter with suction, and add 0.1 g of the crystal obtained in Example 1 as a seed crystal , there is solid precipitation, after stirring for 1 hour, add 200ml of isopropanol dropwise, and cool to 0-5°C after dropping, after stirring for 2 hours, filter with suction, wash the filter cake with isopropanol / water=4:1, at 50-60 ℃, 10mmHg and dried for 1.5 hours to obtain 8.0g of crystals.
[0065] It is detected that: the HPLC purity of the crystal obtained in this embodiment is 99.83%, the residual amount of isopropanol in it is 259ppm, and the moisture measured by the Karl-Fischer (KF) method is 4.81%; and has figure 1 The powder X-ray diffraction spectrum shown, figure 2 The DSC spectrum shown, image 3 The TG spectrum shown and Figure 4 Infrared (IR) spectral features shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com