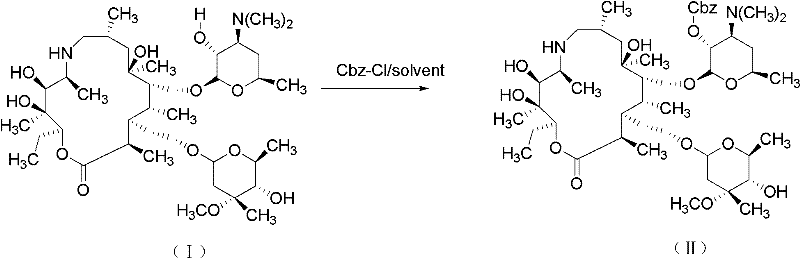A kind of synthetic method of telamectin
A synthesis method and technology of tyramectin, applied in the fields of medicinal chemistry and organic chemistry, can solve the problems of difficult industrial scale-up, failure to achieve effective synthesis, inconvenient operation, etc., and achieve the effects of low raw material cost, simple method and convenient operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
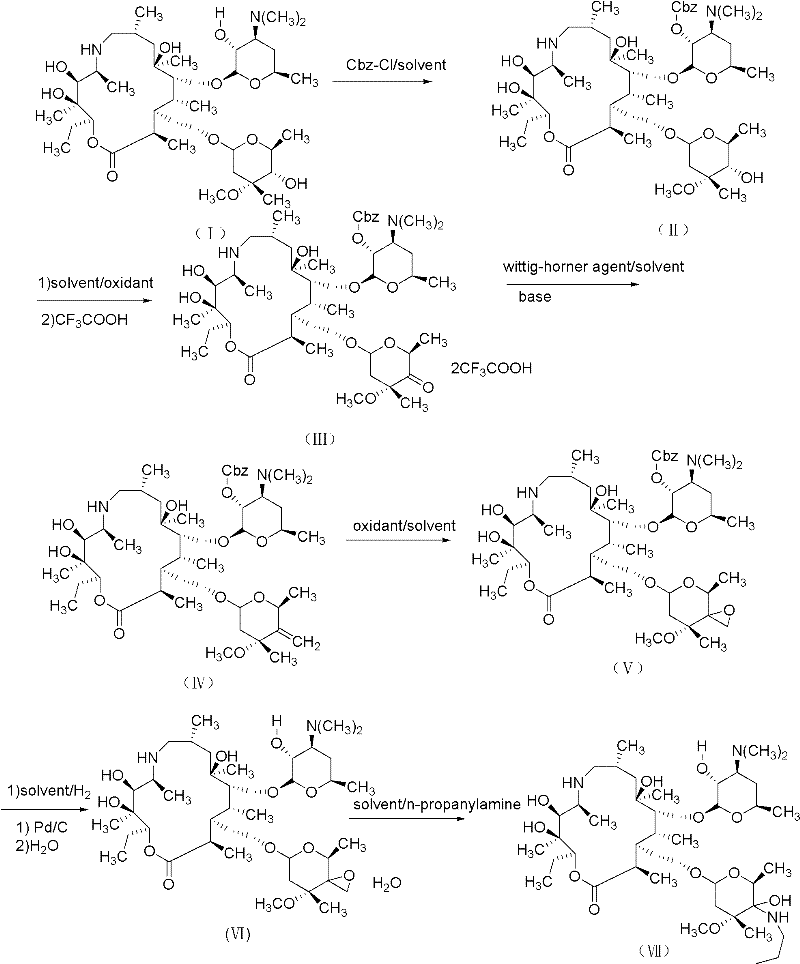
[0060] A kind of synthetic method of telamectin, its step is as follows:
[0061] 1) Preparation of Protected Azithromycin A (II)
[0062] Add 5g of azithromycin A and 75ml of dichloroethane to a 250ml one-mouth bottle, slowly add 2.4ml of benzyl chloroformate dropwise at 0°C, keep the temperature of the reaction solution at 0-5°C during the whole dropping process, and keep it warm for 3h after the drop is complete , the solution was concentrated to 25ml, HPLC analysis contained 5.25 grams of product (yield 94.4%), and the solution was directly used in the next step reaction.
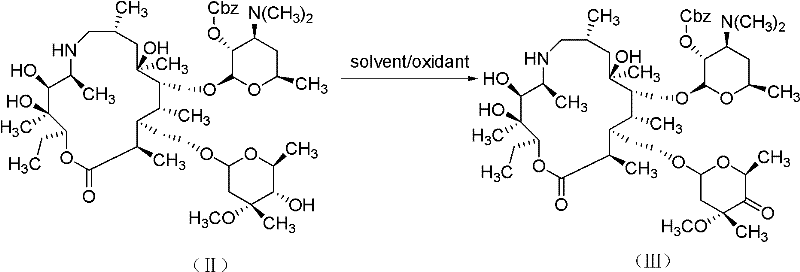
[0063] 2) Preparation of protected ketone (III)
[0064] Add 1.4ml dimethyl sulfoxide (DMSO) to the concentrated solution obtained in the previous step, cool the mixed solution to -10°C with an ice-salt bath, add 2.2 grams of phenyl dichlorophosphate, keep the reaction for half an hour, add 3.44 grams of trichlorophosphate Ethylamine, continue to stir and react for half an hour, add 35ml of water to the...
Embodiment 2
[0083] A kind of synthetic method of telamectin, its step is as follows:
[0084] 1) Preparation of Protected Azithromycin A (II)
[0085] Add 5g (0.033mol) of azithromycin A and 75ml of dichloromethane to a 250ml single-necked bottle, slowly add 2.4ml (0.3mol) of benzyl chloroformate dropwise at 0°C, and maintain the temperature of the reaction solution at 0-5°C throughout the dropping process. After dropping, the reaction was incubated for 3 hours, and the solution was concentrated to 25 ml. HPLC analysis contained 5.15 grams of product (yield 92.6%), and the solution was directly used in the next reaction.
[0086] 2) Preparation of protected ketone (III)
[0087]Add 1.4ml dimethyl sulfoxide (DMSO) to the concentrated solution obtained in the previous step, cool the mixed solution to -10°C with an ice-salt bath, add 2.2 grams of phenyl dichlorophosphate, keep the reaction for half an hour, add 3.44 grams of trichlorophosphate Ethylamine, continue to stir and react for hal...
Embodiment 3
[0100] A kind of synthetic method of telamectin, its step is as follows:
[0101] 1) Preparation of Protected Azithromycin A (II)
[0102] Add 5g of azithromycin A and 75ml of chloroform to a 250ml single-necked bottle, slowly add 2.4ml of benzyl chloroformate dropwise at 0°C, keep the temperature of the reaction solution at 0-5°C during the whole dropping process, keep the temperature for 3h after dropping, and dissolve the solution Concentrated to 25ml, HPLC analysis contained 5.3 grams of product (yield 90.6%), and the solution was directly used in the next reaction.
[0103] 2) Preparation of protected ketone (III)
[0104] Add 1.4ml dimethyl sulfoxide (DMSO) to the concentrated solution obtained in the previous step, cool the mixed solution to -10°C with an ice-salt bath, add 4.2 grams of bis(2-oxo-3-oxazolidinyl) once Phosphorus oxychloride (BOP-Cl), heat preservation reaction for half an hour, add 4.8ml triethylamine, continue to stir and react for half an hour, add 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com