A method for preparing (2s,3s)-2,3-butanediol and (3s)-acetoin from glucose
A technology of butanediol and glucose, applied in the field of biochemical industry, can solve the problems of restricted application, high cost, unsuitable for actual production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: Preparation of Klebsiella pneumoniae CICC 10011 resting cell biocatalyst
[0057] (1) Slant culture: Streak Klebsiella pneumoniae CICC 10011 (purchased from China Industrial Microorganism Culture Collection Management Center) onto a solid medium slant containing 1.5% agar in a mass-volume ratio, and culture at 37°C for 14 hours .
[0058] (2) Seed cultivation: under aseptic conditions, use a sterile inoculation loop to pick a ring of bacteria sludge on the slope of step (1), inoculate into 50 ml of liquid medium, and incubate at 37° C. for 12 hours.
[0059] (3) Culture in a fermenter: Under sterile conditions, inoculate 5 liters of liquid culture medium with the inoculum size of 5% of the culture solution obtained in step (2), and cultivate at 37° C. for 16 hours.
[0060] (4) Collect the cells: Centrifuge the culture obtained in step (3) at 8,000 rpm for 10 minutes, wash the cells twice with pH 7.0 phosphate buffer, and then resuspend the cells in pH 7....
Embodiment 2
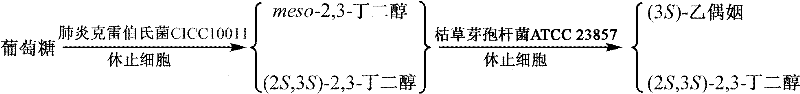
[0063] Embodiment 2: the mixture of (2S, 3S)-2,3-butanediol and meso-2,3-butanediol prepared by using the biocatalyst obtained in Example 1
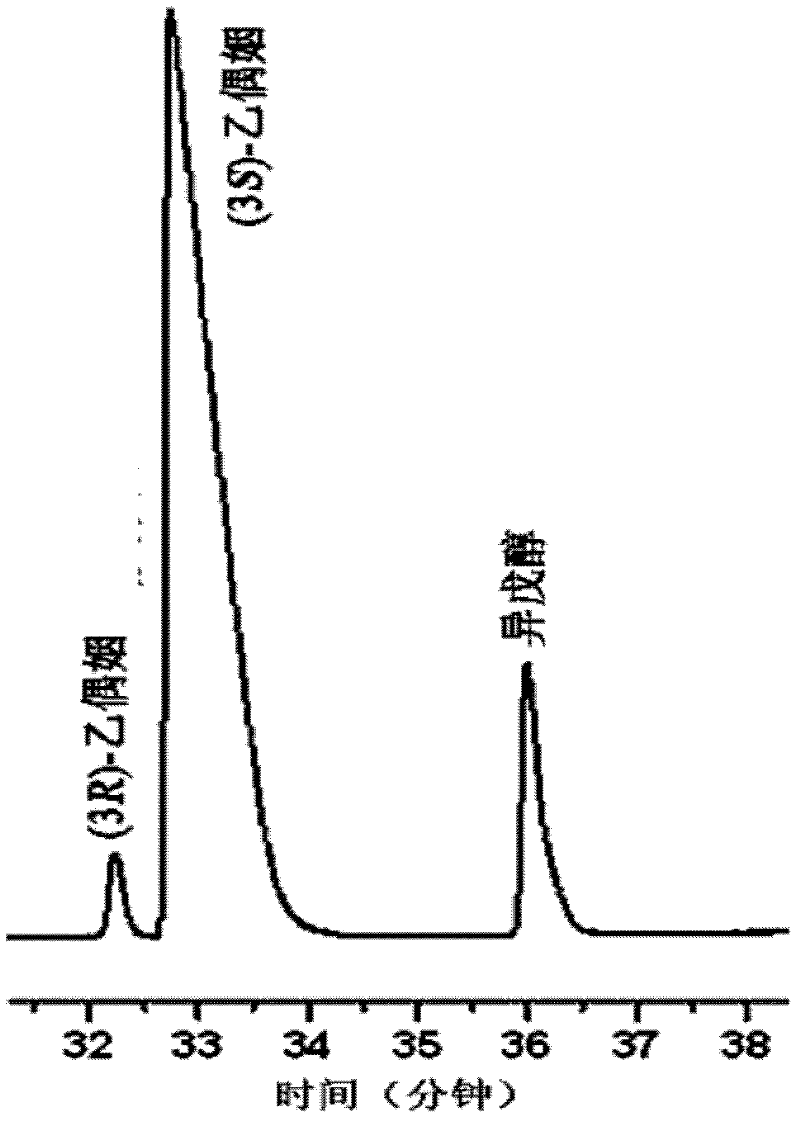
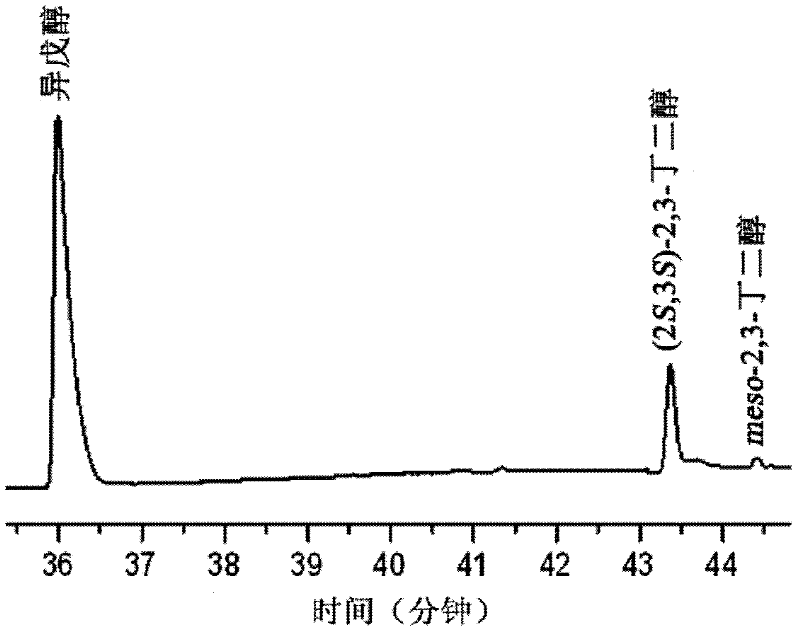
[0064] Transformation experiment: the resulting biocatalyst, Klebsiella pneumoniae CICC 10011, had a cell concentration of 28 g dry cell weight / liter, an initial glucose concentration of 100 g / liter, and reacted at 37°C and pH 6.0. The reaction medium was for distilled water. The reaction was terminated after 8 hours to obtain a conversion solution containing a mixture of (2S,3S)-2,3-butanediol and meso-2,3-butanediol. The resulting conversion liquid was centrifuged at 8,000 rpm for 15 minutes to remove the added biocatalyst, and the concentration of (2S, 3S)-2,3-butanediol in the supernatant was measured by gas chromatography to be 3.2 grams per liter, meso- The 2,3-butanediol concentration was 40.7 g / l.
[0065] Collect the supernatant and carry out vacuum distillation at 50°C and 0.098 MPa, redissolve the fraction with a small amoun...
Embodiment 3
[0066] Embodiment 3: the mixture of (2S, 3S)-2,3-butanediol and meso-2,3-butanediol prepared by using the biocatalyst obtained in Example 1
[0067] Transformation experiment: the obtained biocatalyst, Klebsiella pneumoniae CICC 10011, was reacted at a cell concentration of 36 g dry cell weight / liter and an initial glucose concentration of 110 g / liter at 37° C. and pH 7.0. The reaction was terminated after 6 hours to obtain a conversion solution containing a mixture of (2S,3S)-2,3-butanediol and meso-2,3-butanediol. The resulting conversion liquid was centrifuged at 8,000 rpm for 15 minutes to remove the added biocatalyst, and the concentration of (2S, 3S)-2,3-butanediol in the supernatant was measured by gas chromatography to be 3.0 grams per liter, meso- The 2,3-butanediol concentration was 38.8 g / l.
[0068] Collect the supernatant and carry out vacuum distillation at 50°C and 0.060 MPa, and redissolve the fraction with a small amount of water to obtain a high-concentratio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com