Application of an electrocatalyst in the anode of proton exchange membrane fuel cell
A technology of proton exchange membrane and electrocatalyst, which is applied in battery electrodes, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problems of commercial application gap and achieve fast reduction, Excellent hydrogen oxidation activity, narrow distribution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: get 67.8mL Pd content and be the PdCl of 2.36mg / mL 2 Add hydrochloric acid solution in the three-necked flask, add 300mL ethylene glycol, then add 5mL 37wt% concentrated ammonia water, stir for 5 minutes, the solution becomes colorless, then add 10.1mL Pt content of 3.94mg / mL H 2 PtCl 6 Aqueous solution, then add 800mg Vulcan XC-72R carbon powder, disperse evenly, add 200mL NaBH drop by drop 4 10mg / mL NaBH 4 Aqueous solution, stirred for 3 hours, settled for 40 hours, centrifuged, washed, and dried in a vacuum oven at 60°C for 12 hours to obtain the catalyst 20% Pd 16 Pt 4 / XC-72.
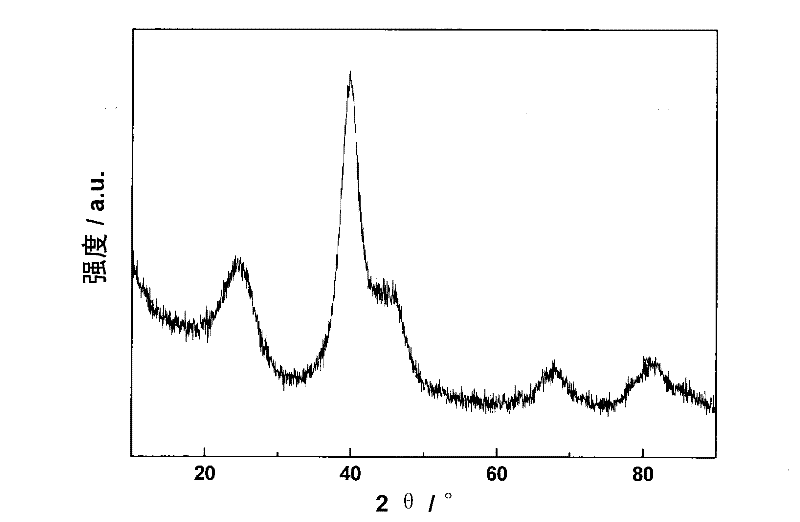
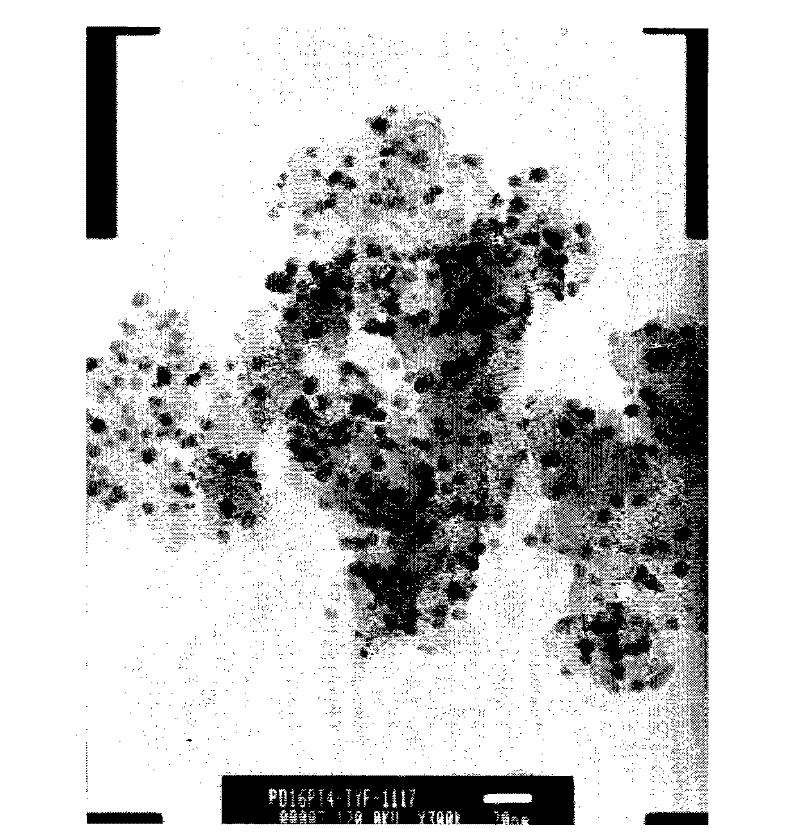
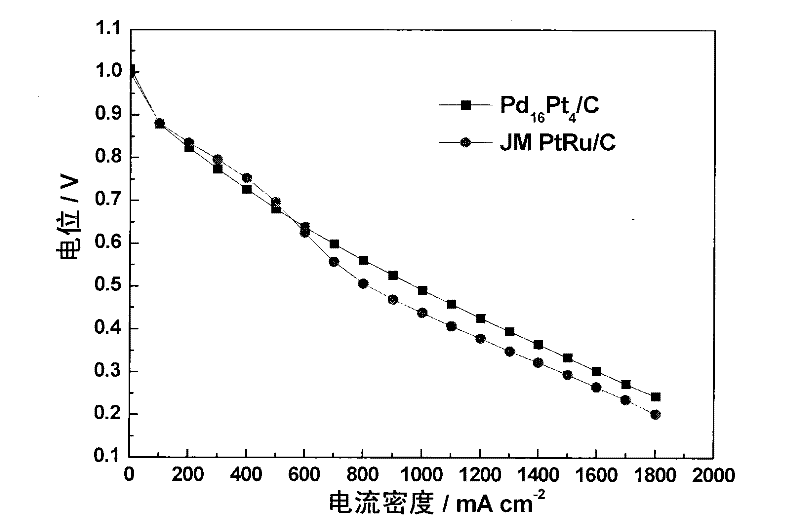
[0039] Depend on figure 1 It can be seen from the XRD that the alloy exists in a face-centered cubic (fcc) structure, without diffraction peaks of oxides, and the grain size calculated by Scherrer's formula is 2.8nm. Depend on figure 2 It can be seen from the TEM that the metal particles of the obtained catalyst are small, and the average particle diameter obtained by s...
Embodiment 2
[0040] Embodiment 2: get 67.8mL Pd content and be the PdCl of 2.36mg / mL 2 Add the hydrochloric acid solution into the three-necked flask, add 300mL of ethylene glycol and 5mL of concentrated ammonia water, stir for 5 minutes, the solution becomes colorless, then add 7.6mL of H2O with a Pt content of 3.94mg / mL 2 PtCl 6 aqueous solution, then add 3.4 mL of RuCl with a Ru content of 2.94 mg / mL 3 Aqueous solution, then add 800mg Vulcan XC-72R carbon powder, disperse evenly, add 200mL NaBH drop by drop 4 10mg / mL NaBH 4 Aqueous solution, stirred for 3 hours, settled for 40 hours, centrifuged, washed, and dried in a vacuum oven at 80°C for 12 hours to obtain the catalyst 20% Pd 16 Pt 3 Ru 1 / XC-72.
Embodiment 3
[0041] Embodiment 3: get 80.5mL Pd content and be the PdCl of 2.36mg / mL 2 Add hydrochloric acid solution into a three-necked flask, add 300mL ethylene glycol, then add 5mL concentrated ammonia water, stir for 5 minutes, the solution becomes colorless, then add 2.5mL H2O with a Pt content of 3.94mg / mL 2 PtCl 6 Aqueous solution, then add 300mg Vulcan XC-72R carbon powder, disperse evenly, add 300mL NaBH drop by drop 4 KBH at 8mg / mL 4 Aqueous solution, stirred for 3 hours, settled for 40 hours, centrifuged, washed, and dried in a vacuum oven at 100°C for 24 hours to obtain the catalyst 40% Pd 19 Pt 1 / XC-72.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com