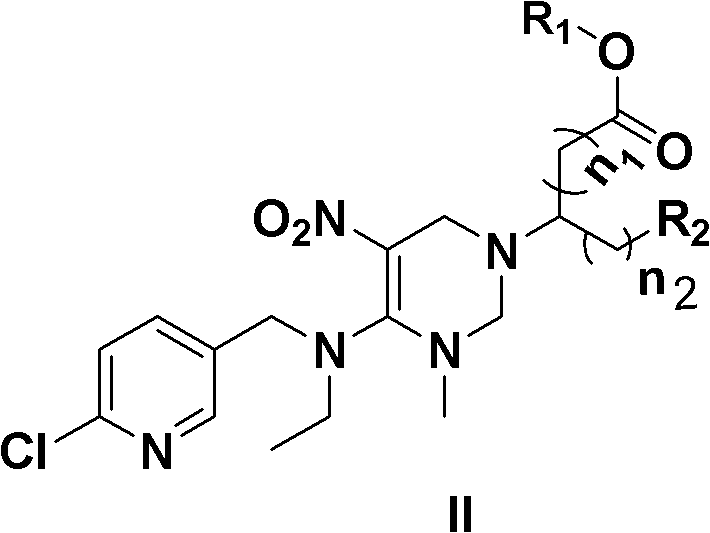
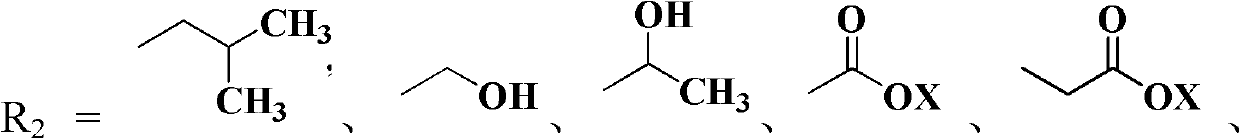
Cis-nitenpyram compound containing amino-acid ester as well as preparation method and application thereof
A technology of amino acid esters and nitenpyram, which is applied in the field of cis-nitenpyram compounds and their preparation, to achieve low toxicity, good insecticidal effect and the effect of promoting the growth of crops
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] cis-(+)-2-[4-N-ethyl-N-(6-chloro-3-pyridylmethyl)amino]-3-methyl-5-nitro-1,2,3,6- Tetrahydropyrimidin-1-yl]-4-carboxamido-butyric acid methyl ester (Ia);
[0055] no 1 =0n 2 = 2 R 1 =CH 3
[0056]Add 20mL of methanol into a 100mL three-necked bottle (with a dry lye absorption device), cool it in an ice-salt bath to -10°C to -15°C, add 3.60mL of freshly steamed thionyl chloride dropwise under magnetic stirring, and control the dropping rate so that The reaction temperature does not exceed -5°C, and after 1 hour of reaction, the temperature is naturally raised to room temperature to obtain a thionyl chloride-methanol solution. Add 1.46 g (0.01 mol) of glutamine to the thionyl chloride-methanol solution, heat to reflux for 1 hour, remove the solvent and excess thionyl chloride under reduced pressure, and proceed directly to the next step without purification.
[0057] The product after the first step reaction and 2.707g nitenpyram (0.01mol), 1.8mL triethylamine (0....
Embodiment 2
[0063] cis-(+)-2-[4-N-ethyl-N-(6-chloro-3-pyridylmethyl)amino]-3-methyl-5-nitro-1,2,3,6- Tetrahydropyrimidin-1-yl]-4-carboxamido-butyric acid ethyl ester (Ib);
[0064] no 1 =0 n 2 = 2 R 1 =CH 2 CH 3
[0065] Add 20mL of ethanol to a 100mL three-necked bottle (with dry lye absorption device), cool in an ice-salt bath to -10°C to -15°C, add 3.60mL of freshly steamed thionyl chloride dropwise under magnetic stirring, and control the dropping rate so that The reaction temperature does not exceed -5°C, and after 1 hour of reaction, the temperature is naturally raised to room temperature to obtain a thionyl chloride-ethanol solution. Add 1.46 g of glutamine (0.01 mol) to the thionyl chloride-ethanol solution, heat to reflux for 1 hour, remove the solvent and excess thionyl chloride under reduced pressure, and proceed directly to the next step without purification.
[0066] The product after the first step reaction and 2.707g nitenpyram, 1.8mL triethylamine (0.012mol), and ...
Embodiment 3
[0072] cis-(+)-2-[4-N-ethyl-N-(6-chloro-3-pyridylmethyl)amino]-3-methyl-5-nitro-1,2,3,6- Tetrahydropyrimidin-1-yl]-4-carboxamido-butyric acid n-propyl ester (Ic);
[0073] no 1 =0n 2 = 2 R 1 =CH 2 CH 2 CH 3
[0074] Add 20mL of n-propanol into a 100mL three-necked bottle (with a dry lye absorption device), cool it in an ice-salt bath to -10°C~-15°C, add 3.60mL of freshly distilled thionyl chloride dropwise under magnetic stirring, and control the dropwise The acceleration keeps the reaction temperature not exceeding -5°C, and after 1 hour of reaction, the temperature is naturally raised to room temperature to obtain a thionyl chloride-n-propanol solution. Add 1.46 g of glutamine (0.01 mol) to the thionyl chloride-n-propanol solution, heat to reflux for 1 hour, remove the solvent and excess thionyl chloride under reduced pressure, and proceed directly to the next step without purification.
[0075] The product after the first step reaction and 2.707g nitenpyram (0.01m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com