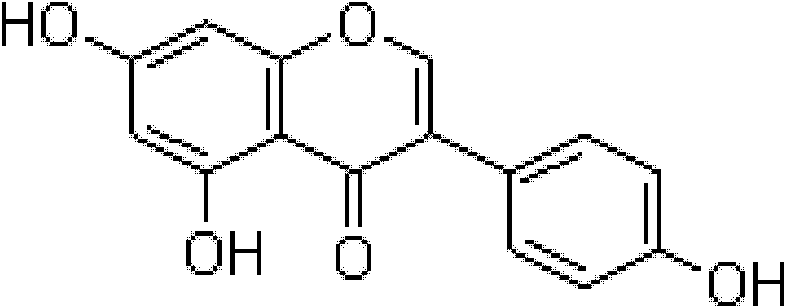
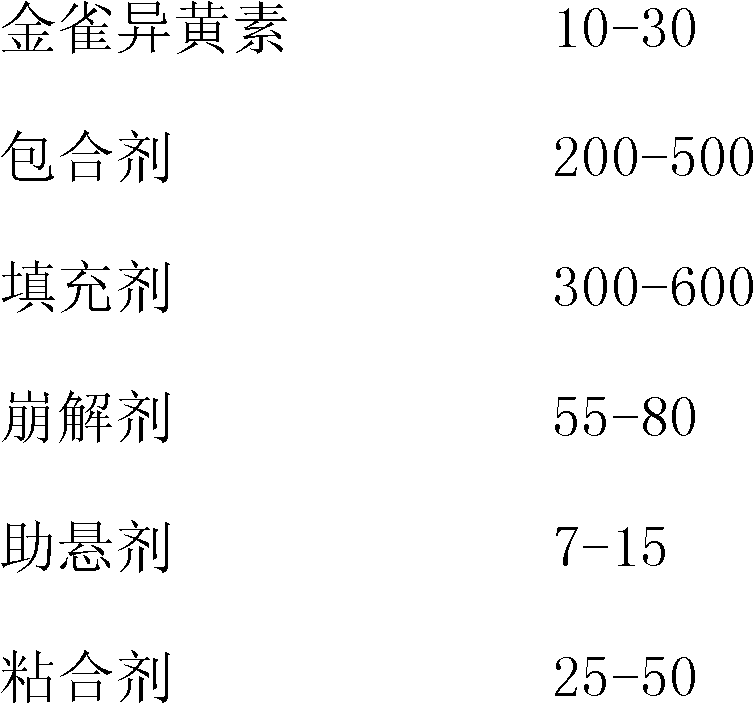
Genistein clathrate compound-containing preparation and its preparation method and use
A technology of genistein and clathrate, applied in the field of medicine, can solve the problems of increasing the risk of adverse reactions, limiting the types of preparations, increasing the cost of medication, etc., and achieving easy industrial production, rapid curative effect, economical and social High benefit effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Example 1: Preparation of genistein freeze-dried powder for injection
[0109] Weigh 2.0g of genistein, 60.0g of hydroxypropyl-β-cyclodextrin, and 124g of mannitol, first dissolve genistein in 130ml of ethanol, stir to dissolve, then add hydroxypropyl-β-cyclodextrin Refining, magnetic stirring for 2 hours, temperature 50°C, after clathrate, remove ethanol under reduced pressure, add mannitol and water for injection 1000ml and stir to dissolve, add water for injection to 2000ml full amount, adjust pH to 3.3 with citric acid, add drug Stir and adsorb with activated carbon for 15 minutes, the amount of medicinal carbon used is 0.03%, filter, the filtrate is divided into vials, freeze-dried, vacuum-pressed and taken out, and the aluminum-pressed cap is labeled to obtain the freeze-dried product.
Embodiment 2
[0110] Embodiment 2: Preparation of Genistein Sodium Chloride Injection
[0111]Weigh 40.0g of hydroxypropyl-β-cyclodextrin, 1.0g of genistein and 9.0g of sodium chloride for injection, add genistein to 60ml of ethanol, stir to dissolve and add hydroxypropyl -β-cyclodextrin and sodium chloride for injection, magnetically stirred for 1 hour (control the speed so that the liquid does not splash outwards), temperature 60°C, observed to obtain clear and transparent genistein hydroxypropyl-β-cyclodextrin clathrate solution. Remove ethanol under reduced pressure, add water for injection to 600ml and stir, add water for injection to 1000ml full amount, adjust pH to 3.8 with tartaric acid, add medicinal active carbon and stir for adsorption for 20 minutes, the amount of medicinal carbon used is 0.03%, filter, press The predetermined dose is divided into infusion bottles and sterilized by autoclaving at 115°C for 30 minutes.
Embodiment 3
[0112] Embodiment 3: Preparation of Genistein Glucose Injection
[0113] Weigh 50.0g of hydroxypropyl-β-cyclodextrin, 2.0g of genistein, and 50g of glucose for injection, add genistein to 150ml of ethanol, stir and dissolve, then add hydroxypropyl-β- Cyclodextrin, stirred by magnetic force for 1 hour (the speed is controlled so that the liquid does not splash outside), the temperature is 55° C., and a clear and transparent genistein hydroxypropyl-β-cyclodextrin inclusion complex solution is obtained. Ethanol was removed under reduced pressure. After glucose for injection and 100ml of water for injection are stirred and dissolved, the glucose solution is poured into the clathrate, replenished to 800ml, adjusted to pH 3.8 with tartaric acid, added with medicinal activated carbon and stirred and adsorbed for 20 minutes. The amount of medicinal carbon used is 0.03%, filtered, divided into infusion bottles according to the predetermined dose, and sterilized at 115°C for 30 minutes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com