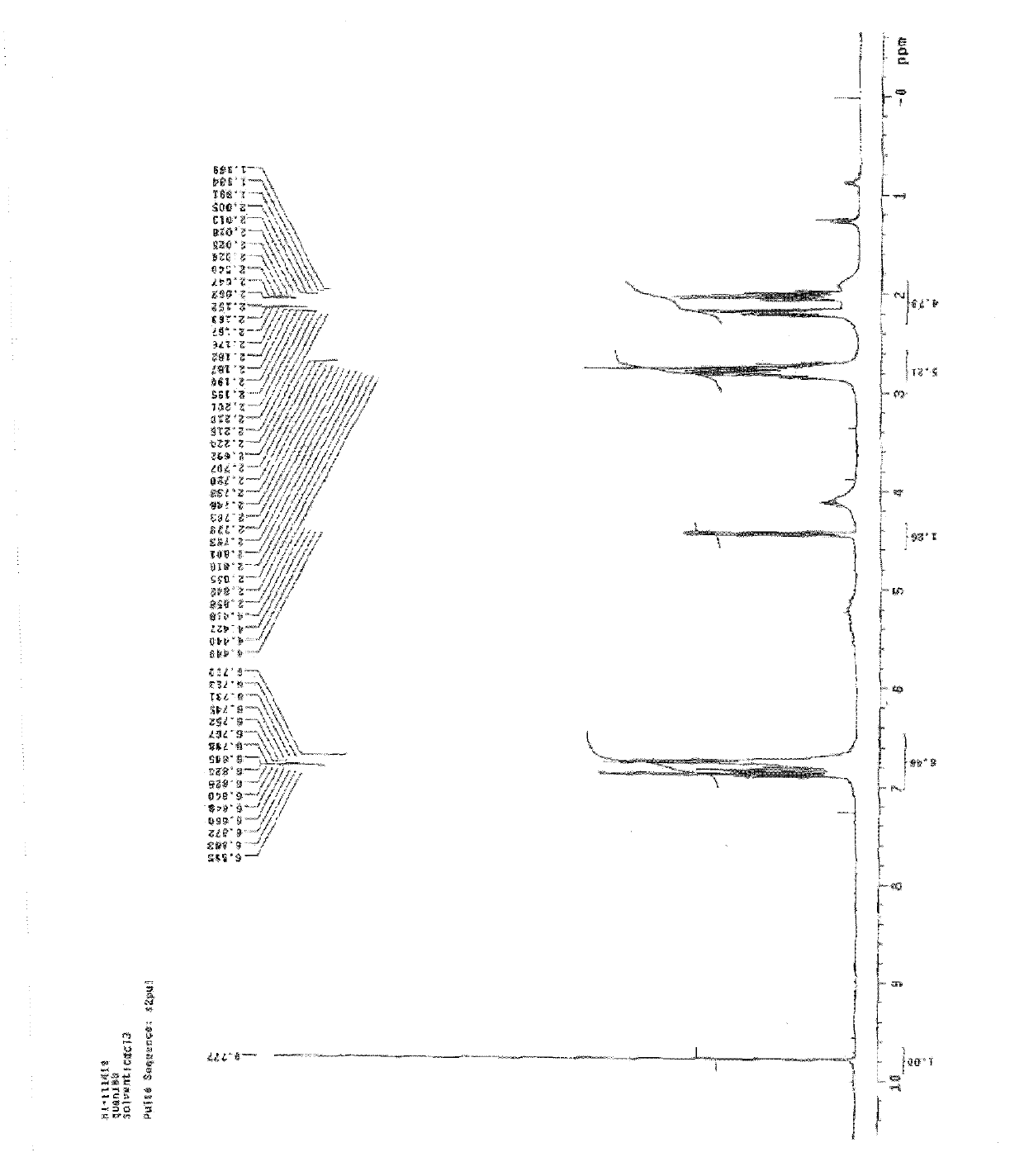
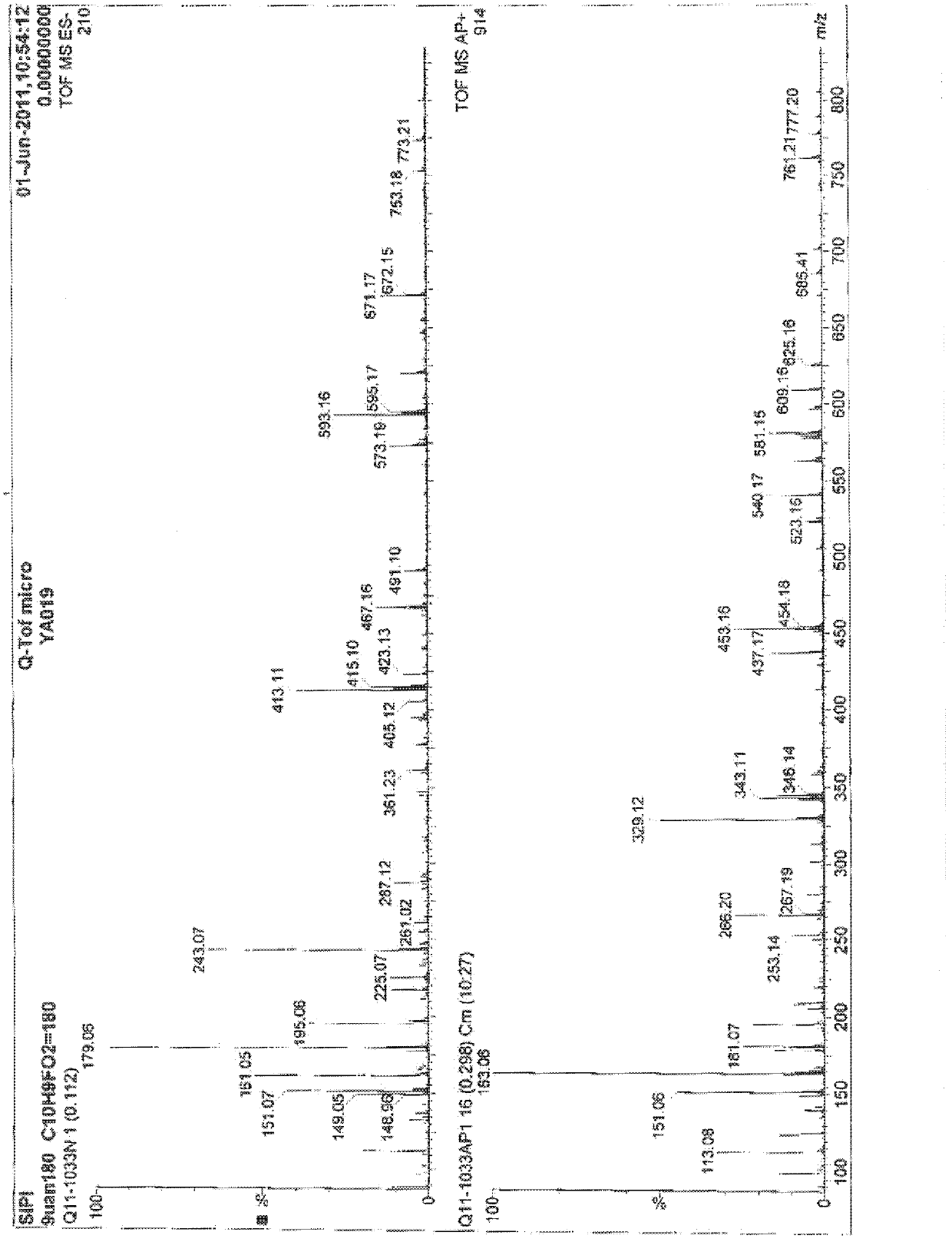
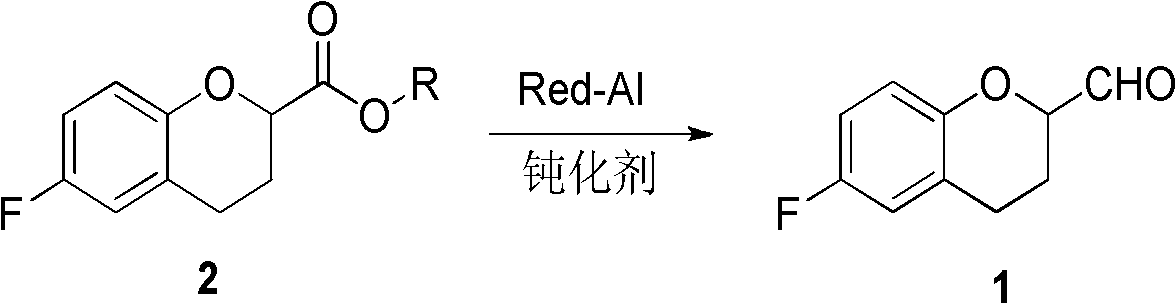
Method for preparing 6-fluoro-3,4-dihydro-2H-1-benzopyran-2-formaldehyde
A technology of benzopyran and dihydro, applied in the field of preparation of 6-fluoro-3,4-dihydro-2H-1-benzopyran-2-carbaldehyde, can solve the problem of harsh conditions, complex operation, reagent Risk and other problems, to achieve the effect of mild reaction conditions, cheap and easy to obtain reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Under nitrogen protection, 12.8ml of reducing agent (toluene solution (content 70%) of dihydrobis(2-methoxyethoxy)sodium aluminate was added in the reaction flask, 15ml of toluene was added for dilution, and the temperature was lowered to 0°C, slowly add 3.97g of equivalent morpholine dropwise to it, keep the temperature at 0-5°C, after the dropwise addition is completed, raise the temperature to room temperature, and after 4 hours of reaction, slowly add the reaction solution dropwise to the raw material 6-fluoro -3,4-dihydro-2H-1-benzopyran-2-methyl ester 8.0g in 40ml toluene solution, the temperature is raised during this process, and the temperature is maintained at -15~-5°C. After the dropwise addition is completed, the reaction 4 hour, the reaction solution was added to 60ml of ice-water solution, stirred for 15 minutes, and the liquid was separated. The organic layer was washed twice with 50ml of salt water. After the liquid separation, the organic layer was dried...
Embodiment 2
[0023] Under nitrogen protection, 8.0ml of reducing agent (toluene solution of dihydrobis(2-methoxyethoxy)sodium aluminate (content 70%) was added in the reaction flask, 10ml of toluene was added for dilution, and the temperature was lowered to 0°C, slowly add 2.03g of tetrahydropyrrolidine equivalent to it dropwise, maintain the temperature at 0-5°C, after the dropwise addition, raise the temperature to room temperature, and slowly add the reaction solution dropwise to the raw material 6 after 4 hours of reaction -Fluoro-3,4-dihydro-2H-1-benzopyran-2-ethyl ester 5.0g in 30ml of toluene solution, the temperature is raised during this process, and the temperature is maintained at -15--5°C. After the dropwise addition is completed, After reacting for 4 hours, the reaction solution was added to 50 ml of ice-water solution, stirred for 15 minutes, and separated. The organic layer was washed twice with 400 ml of salt water. After the liquid separation, the organic layer was dried an...
Embodiment 3
[0025] Under nitrogen protection, 8.0ml of reducing agent (toluene solution of dihydrobis(2-methoxyethoxy)sodium aluminate (content 70%) was added in the reaction flask, 10ml of toluene was added for dilution, and the temperature was lowered to 0°C, slowly add 1.74g of isopropanol equivalent to it dropwise, maintain the temperature at 0-5°C, after the dropwise addition, raise the temperature to room temperature, and slowly add the reaction solution dropwise to the raw material 6- Fluorine-3,4-dihydro-2H-1-benzopyran-2-methyl ester 5.0g in 40ml toluene solution, the temperature is raised during this process, and the temperature is maintained at -15--5°C. After the dropwise addition is completed, the reaction After 6 hours, the reaction solution was added to 40 ml of ice-water solution, stirred for 15 minutes, and separated. The organic layer was washed twice with 30 ml of salt water. After the liquid separation, the organic layer was dried and evaporated to dryness to obtain the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com