Prophylactic/therapeutic agent for cancer
A therapeutic agent, cancer technology, applied in pill delivery, medical preparations containing active ingredients, animal/human proteins, etc., can solve the problem of undiscovered androgen-independent prostate cancer, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific example
[2886] Specific examples are: (1) EGF (epidermal growth factor) or substances having substantially the same activity as EGF [for example, EGF, nerve growth factor (HER ligand), etc.], (2) insulin or substances having substantially the same activity as insulin A substance with the same activity [for example, insulin, IGF (insulin-like growth factor)-1, IGF-2, etc.], (3) FGF (fibroblast growth factor) or a substance with substantially the same activity as FGF [for example, Acidic FGF, basic FGF, KGF (keratinocyte growth factor), FGF-10, etc.], (4) other cell growth factors [eg, CSF (colony stimulating factor), EPO (erythropoietin), IL-2 (interleukin-2), NGF (nerve growth factor), PDGF (platelet-derived growth factor), TGFβ (trans-transforming growth factor-β), HGF (hepatocyte growth factor), VEGF (vascular endothelial growth factor), etc.
[2887] As long as it can be combined with the above-mentioned cell growth factor, the "receptor of cell growth factor" can be any receptor,...
Embodiment
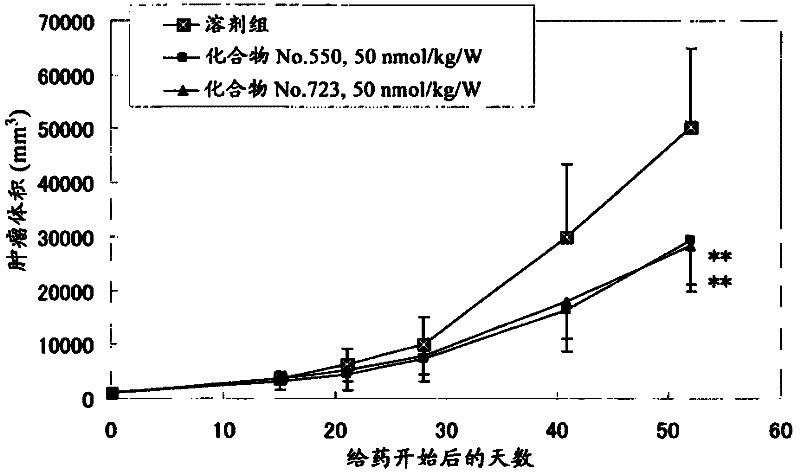
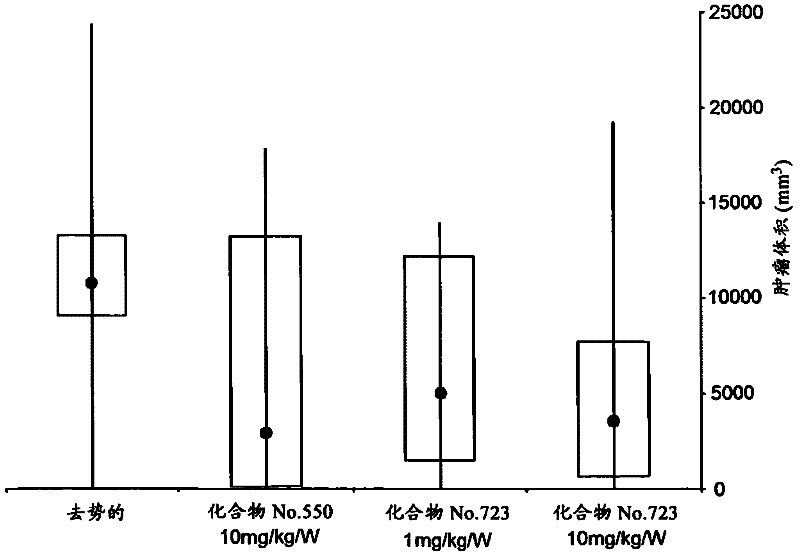
[2949] The present invention will be further illustrated by the following preparation examples and test examples. The present invention is not limited to the above examples, and various changes and corrections are included in the present invention as long as they do not deviate from the spirit and scope of the present invention.
[2950] In the following examples, "room temperature" generally refers to about 10°C to about 35°C. The symbol "%" used hereinafter means mol / mol% with respect to yield, vol% with respect to the solvent used in chromatography, and elsewhere with wt%. In proton NMR spectra, results for broad and undetermined protons such as OH and NH are not mentioned in the data.
[2951] Abbreviated description
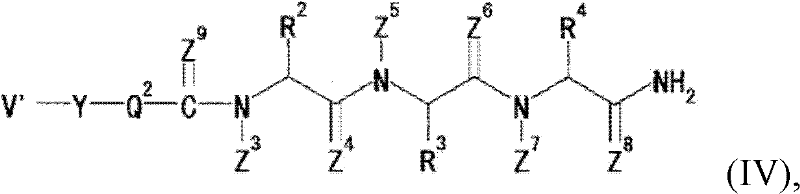
[2952] 10Ψ, CSNH: C-terminal -CONH at the 10-position 2 By-CSNH 2 replace.
[2953] 1Ψ2,CH 2 NH: The -CONH- bond between the 1- and 2-positions is replaced by -CH 2 NH-bond substitution.
[2954] 2Ψ3,CH 2 NH: -CONH-bond between 2- and 3-positions re...
preparation example 1
[3195] (1) Compound No.550 10.0mg
[3196] (2) Lactose 60.0mg
[3197] (3) Corn starch 35.0mg
[3198] (4) Gelatin 3.0mg
[3199] (5) Magnesium stearate 2.0mg
[3200] Using 0.03 ml of 10% aqueous gelatin solution (containing 3.0 mg gelatin), a mixture containing 10.0 mg of Compound No. 550, 60.0 mg of lactose and 35.0 mg of corn starch was granulated through a sieve with a 1 mm mesh at 40° C. Dry and pass through a sieve again. The prepared granules were mixed with 2.0 mg of magnesium stearate and compressed into tablets. The resulting plain tablets were sugar coated with an aqueous suspension of sucrose, titanium dioxide, talc and gum arabic. The obtained coated tablets were then polished with beeswax to obtain coated tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com