Production method of di(tert-butylperoxy)ketal
A technology of tert-butyl peroxyl and tert-butyl hydroperoxide, which is applied in the field of bisketal production, can solve the problems of low product content, unsafe production, severe side reactions, etc., and achieve high product content and safe production High performance and smooth response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
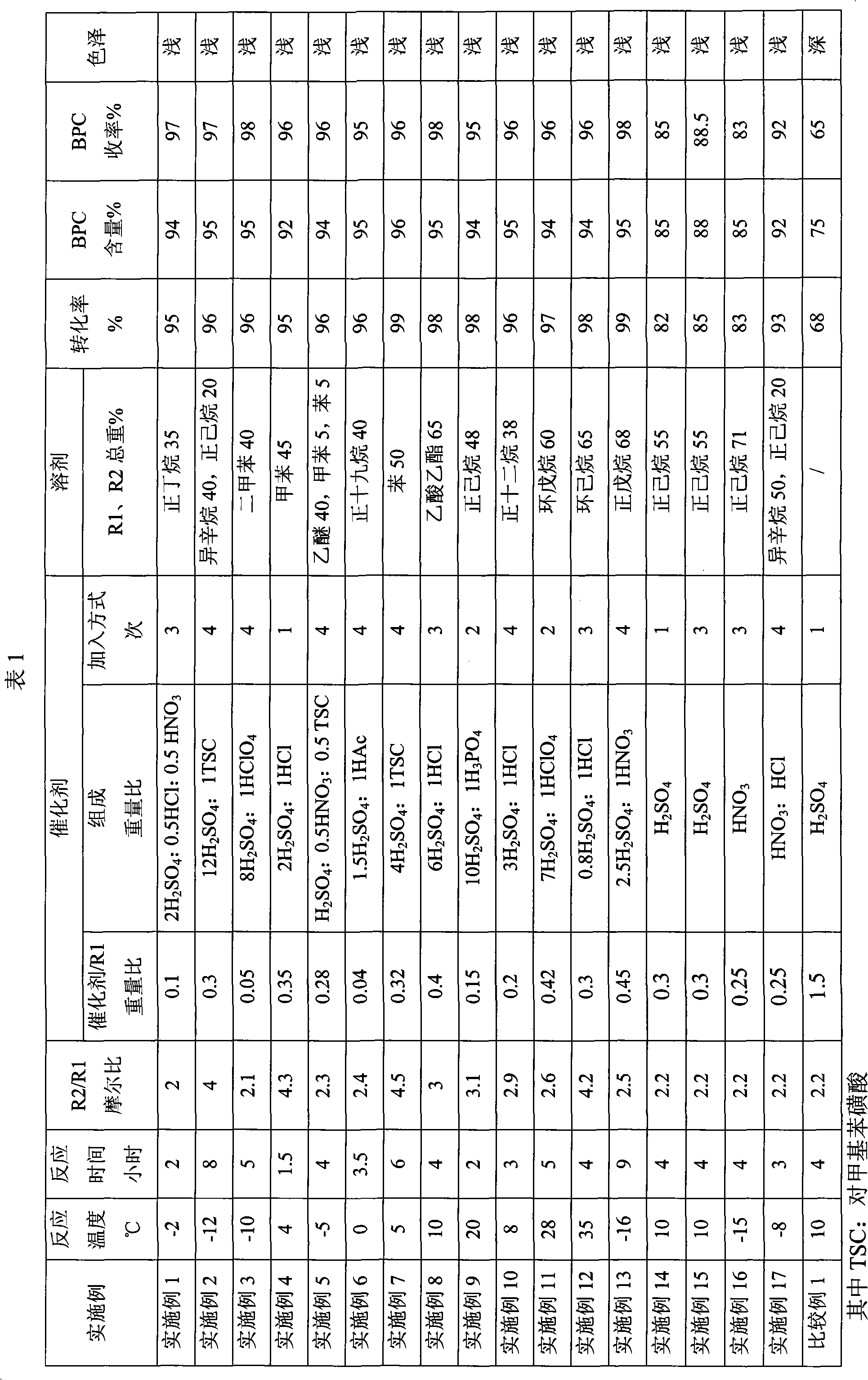
Embodiment 1~17
[0014] Preparation of 1,1-bis(tert-butylperoxy)cyclohexane (BPC)
[0015] Under normal pressure, add the solvent, cyclohexanone R1 and tert-butyl hydroperoxide R2 into a reaction kettle equipped with an electric stirrer, a thermometer, and a reflux condenser. Start the stirring, add the catalyst, and react for 4 hours to obtain the BPC reaction solution. After alkali washing and water washing, a neutral reaction solution is obtained, and a colorless liquid is obtained by vacuum distillation, which is the BPC product. The proportioning of each raw material, the composition, content, adding method of catalyst, the kind of solvent, consumption, reaction temperature, reaction time and content, yield of product, see Table 1 specifically.
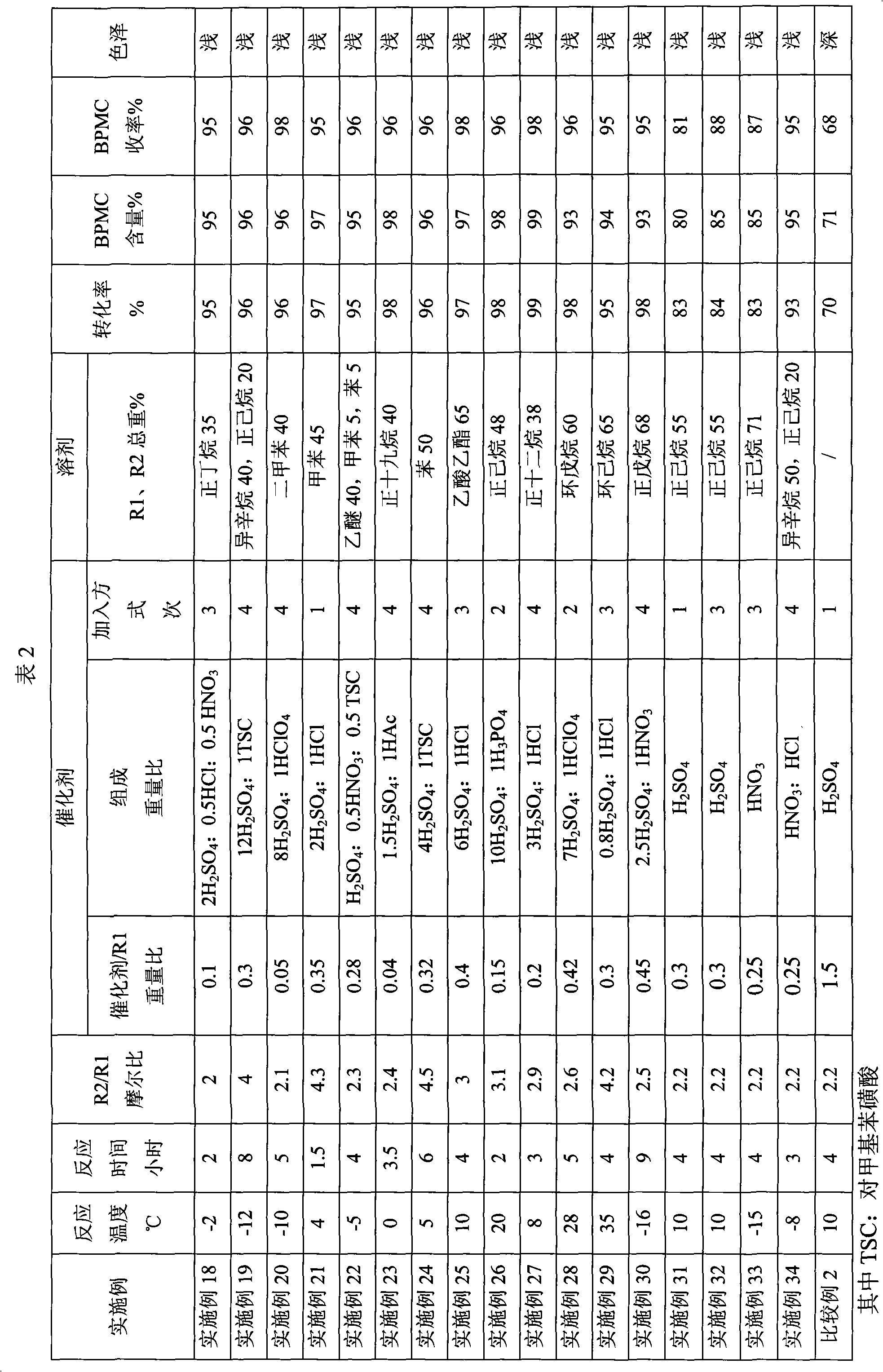
Embodiment 18~34
[0019] Preparation of 1,1-bis(tert-butylperoxy)-3,3,5-trimethylcyclohexane (BPMC)
[0020] Under normal pressure, add the solvent, 3,3,5-trimethylcyclohexanone R1 and tert-butyl hydroperoxide R2 into a reaction kettle equipped with an electric stirrer, a thermometer and a reflux condenser. Start stirring, add the catalyst, and react for 4 hours to obtain the BPMC reaction liquid. After alkali washing and water washing, a neutral reaction solution is obtained, and a colorless liquid is obtained through vacuum distillation, which is the BPMC product. The proportioning of each raw material, the composition, content, adding method of the catalyst, the type and amount of the solvent, the reaction temperature and the reaction time are specifically shown in Table 2.
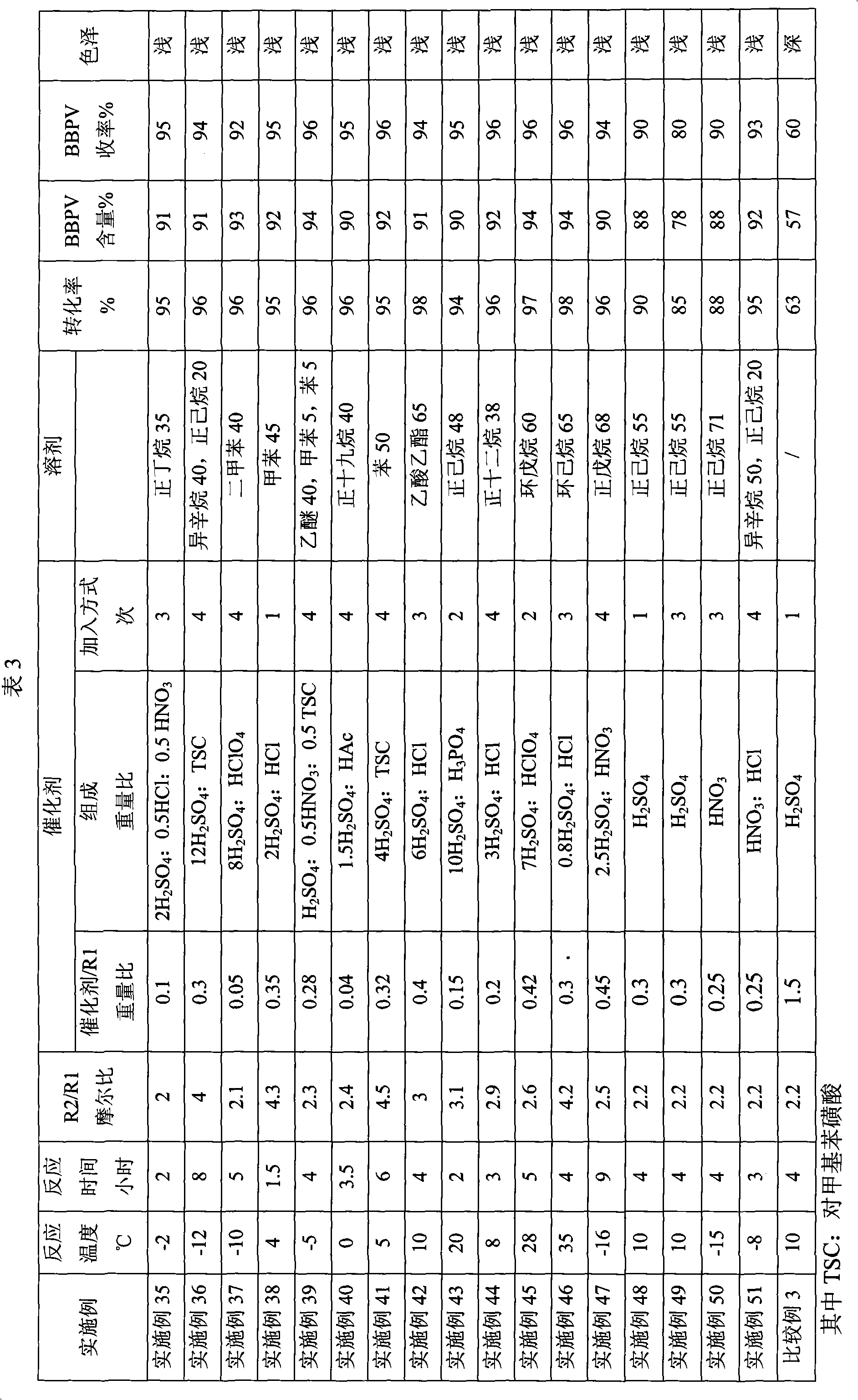
Embodiment 35~51
[0024] Preparation of n-butyl 4,4-bis(tert-butylperoxy)valerate (BBPV)
[0025]Under normal pressure, add the solvent, butyl levulinate R1 and tert-butyl hydroperoxide R2 into a reaction kettle equipped with an electric stirrer, a thermometer, and a reflux condenser. Start the stirring, add the catalyst, and react for 4 hours to obtain the BBPV reaction solution. After alkali washing and water washing, a neutral reaction solution is obtained, and a colorless liquid is obtained by vacuum distillation, which is the BBPV product. The proportioning of each raw material, the composition, content and adding method of the catalyst, the type and amount of the solvent, the reaction temperature and the reaction time are specifically shown in Table 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| activation energy | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| critical temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com