Hydroborate hydrolysis catalyst for preparing hydrogen and its preparation method
A technology for producing hydrogen and borohydride by hydrolysis, which is applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc. It can solve the problem of wide particle size distribution and uneven particle distribution , Low catalytic efficiency and other issues, to achieve the effect of low cost, uniform particle size distribution and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
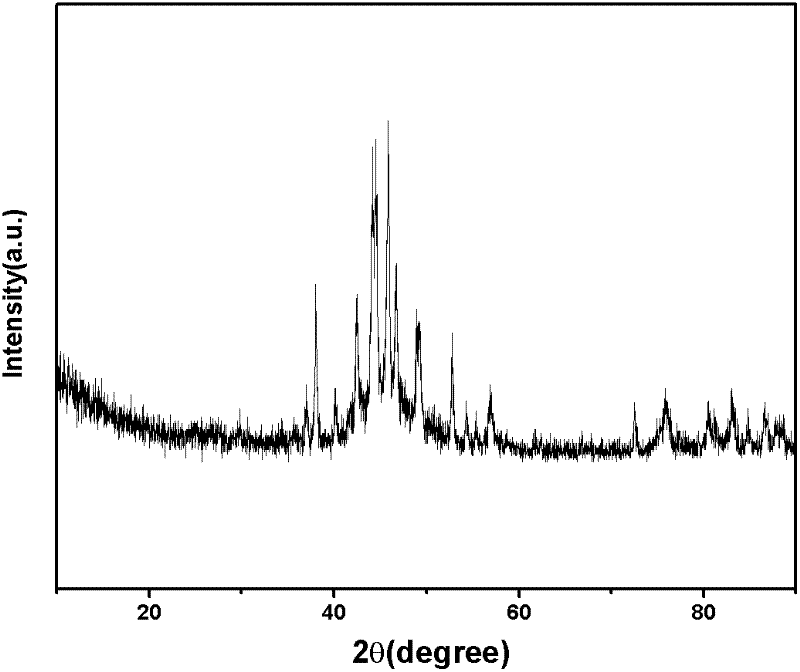
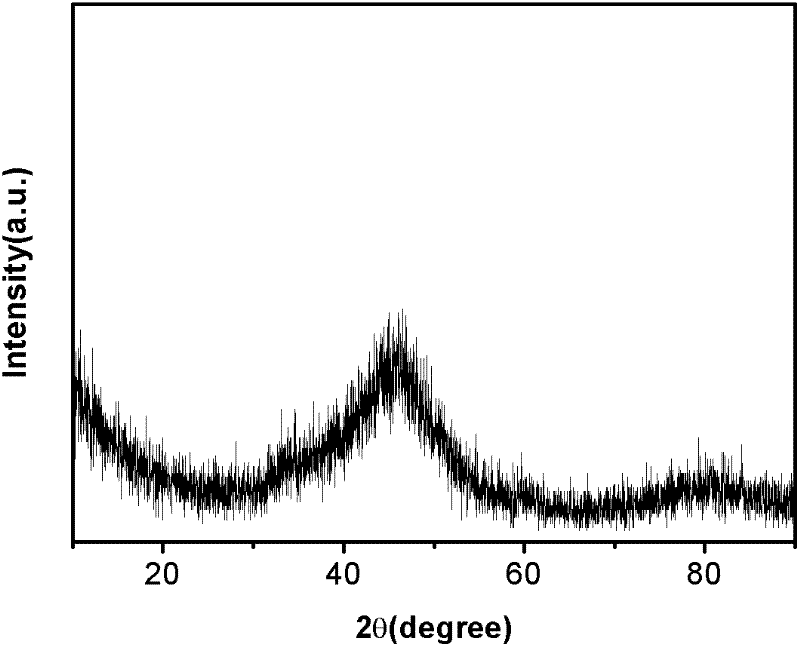
Image
Examples
Embodiment 1
[0040] Place the reactor in an environment of 4°C, under the stirring condition of a magnetic stirrer, add 0.1mol / L, 100mL of CoCl 2 The solution was added to the reactor. Then, at a rate of 1 mL / min, add excess NaBH with NaOH to adjust the pH value to 12 dropwise. 4 Solution (0.5mol / L, 100mL), continued to stir for 0.5h after the dropwise addition to ensure complete reaction, and precipitate 1 was obtained. The obtained precipitate 1 was washed three times with deionized water and then suction-filtered to obtain precipitate 2. Put the precipitate 2 into a cold trap, drop to -90°C together with the cold trap, and freeze at -90°C for 3 hours at a cooling rate of 15°C / min. After freezing, vacuumize to sublimate the liquid in the sediment 2 to realize the drying process. Under the conditions of vacuum degree ≤ 10.0Pa (the vacuum degree can be maintained at ≤ 10.0Pa under the technical conditions of this experiment, the vacuum degree is constantly changing in the actual experim...
Embodiment 2
[0042] Place the reactor in an environment of 4°C, under the stirring condition of a magnetic stirrer, add 0.1mol / L, 100mL of CoCl 2 The solution was added to the reactor. Then, at a rate of 5 mL / min, add excess NaBH with NaOH to adjust the pH value to 12 dropwise. 4 solution (0.1mol / L, 100mL), continue to stir for 0.5h after the dropwise addition to ensure the completion of the reaction, drive away the hydrogen bubbles, and obtain precipitate 1. The obtained precipitate 1 was washed three times with deionized water and then suction-filtered to obtain precipitate 2. Put the precipitate 2 into a cold trap, drop to -90°C together with the cold trap, and freeze at -90°C for 3 hours at a cooling rate of 15°C / min. After freezing, vacuumize to sublimate the liquid in the sediment 2 to realize the drying process. Under the conditions of vacuum degree ≤ 10.0Pa (the vacuum degree can be kept at ≤ 10.0Pa under the technical conditions of this experiment, the vacuum degree is constant...
Embodiment 3
[0044] Place the reactor in an environment of -8°C, under the stirring condition of a magnetic stirrer, add 0.1mol / L, 100mL of CoCl 2 The solution was added to the reactor. Then, at a rate of 5 mL / min, add excess NaBH with ammonia water to adjust the pH value to 12 dropwise. 4 solution (0.5mol / L, 100mL), continue to stir for 0.5h after the dropwise addition to ensure the completion of the reaction, drive away the hydrogen bubbles, and obtain precipitate 1. The obtained precipitate 1 was washed three times with absolute ethanol and once with deionized water, and then suction-filtered to obtain precipitate 2, which was placed in a refrigerator at -4°C for 1 hour. The temperature of the cold trap was lowered to -90°C, and the precipitate 2 placed in the -4°C refrigerator for 1 hour was put into the cold trap, and frozen at -90°C for 6 hours. After freezing, vacuumize to sublimate the liquid in the sediment 2 to realize the drying process. Under the conditions of vacuum degree ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com