Photoreactive Polymer And Preparation Method Thereof
A technology of photoreactivity and polymer, which is applied in the field of alignment layer, can solve the problems of liquid crystal display device contrast degradation, insufficient orientation, slow photoreaction speed, etc., and achieve improved photoreaction speed, improved production efficiency, and excellent thermal stability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0092] On the other hand, another embodiment of the present invention provides a method for preparing the photoreactive polymer. An embodiment of the preparation method includes, in the presence of a catalyst composition comprising a precatalyst having a Group 10 transition metal and a cocatalyst, addition polymerizing a monomer represented by Chemical Formula 1 to form a repeating unit represented by Chemical Formula 3 A step of.
[0093] [chemical formula 1]
[0094]
[0095] In the chemical formula 1, p, R 1 , R 2 , R 3 and R 4 as defined in Chemical Formula 3.
[0096] At this time, the polymerization reaction may be performed at a temperature of 10°C to 200°C. If the reaction temperature is lower than 10°C, the polymerization activity will be reduced, and if it is higher than 200°C, the catalyst will be decomposed, so it is not preferable.
[0097]Also, the cocatalyst may comprise a first cocatalyst selected from the group consisting of a first cocatalyst for pr...
Embodiment 1
[0131] : aggregation of
[0132] In a 250mL Schlenk (Schlenk) flask, add 1.26g (3mmol) and 3 mL of toluene refined with solvent. Then in the flask, 6.73㎎Pd(OAc) dissolved in 1 mL of dichloromethane was added as a catalyst 2 and 7.76 mg of tricyclohexylphosphine, 6.53 mg of dimethylanilinium tetrakiss (pentafluorophenyl) borate was added as a cocatalyst, and the reaction was stirred at 90° C. for 18 hours.
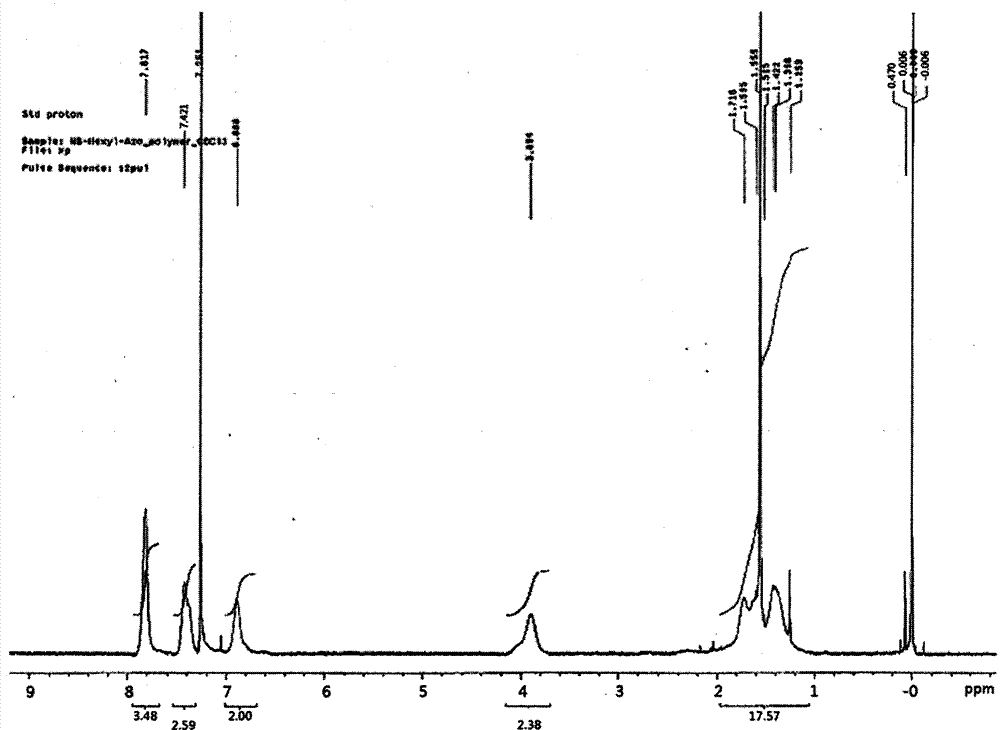
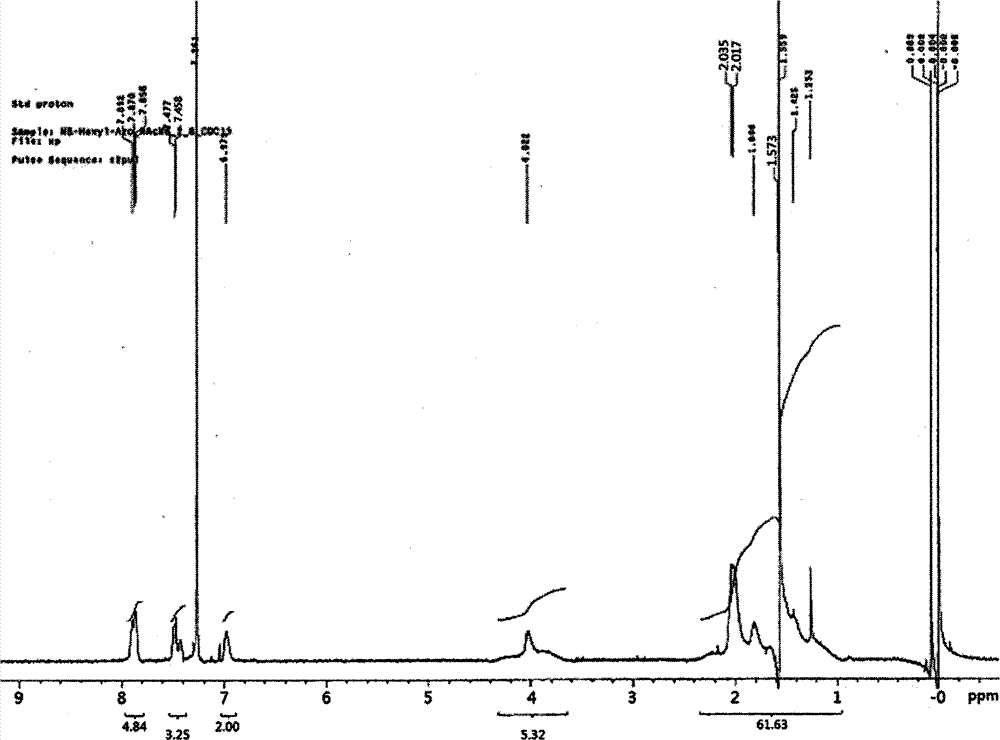
[0133] After reacting for 18 hours, the reactant was put into excess ethanol to obtain a white polymer precipitate. The precipitate was filtered with a glass funnel to collect the polymer, and the collected polymer was dried in a vacuum oven at 60° C. for 24 hours to obtain 1.19 g of polymer (Mw=31,000; PDI=1.7; yield=94%). The 1HNMR data of the polymkeric substance that makes in embodiment 1 is expressed in figure 1 middle.
Embodiment 2
[0134] : aggregation of
[0135] In a 250 mL schlenk flask, add 3.0 g (6.69 mmol) as a monomer and 4 mL of toluene refined with solvent. Then in the flask, 0.75㎎Pd(OAc)2 and 0.86mg tricyclohexylphosphine dissolved in 1mL of dichloromethane were added as a catalyst, and 0.72mg dimethylaniliniumtetrakis(pentafluorophenyl)boron was added as a cocatalyst. Acid acid, stirred and reacted at 90°C for 18 hours.
[0136] After reacting for 18 hours, the reactant was put into excess ethanol to obtain a white polymer precipitate. Use a glass funnel to filter the precipitate to collect the polymer, and dry the collected polymer in a vacuum oven at 60° C. for 24 hours to obtain 2.55 g of polymer (Mw=56,000; PDI=1.9; yield=85%) .
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com