Method for measuring nickel content in low alloy steel by spectrophotometry
A spectrophotometric, low-alloy steel technology, applied in color/spectral characteristic measurement, analysis through chemical reaction of materials, material analysis through observation of the influence of chemical indicators, etc., can solve the difficulty of ensuring accurate measurement results Accuracy and precision, interference and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 Weigh 0.5000 g of the sample (standard sample 1#), accurate to 0.0001 g.
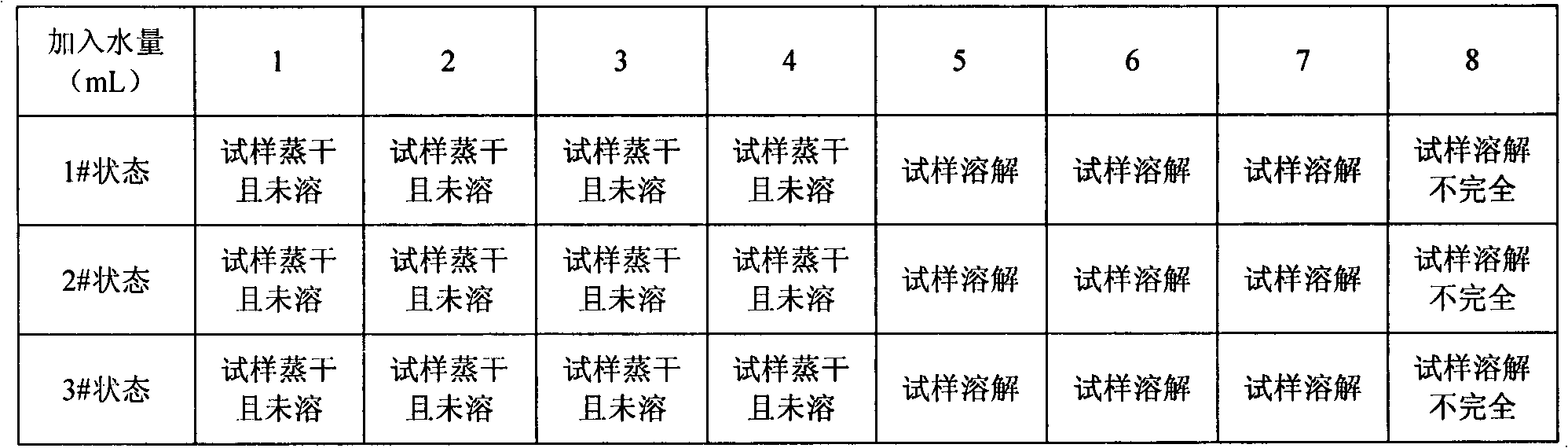
[0054] Put the sample to be weighed in a 100mL steel measuring bottle, add 5mL of a mixture of concentrated hydrochloric acid and concentrated nitric acid, heat, and when the sample is nearly dissolved, add distilled water in portions, 1mL each time, until small bubbles in the solution can be observed Stop adding distilled water until the bubbles disappear and start to appear. At this time, add a total of 5 mL of distilled water, and continue heating until the sample is completely dissolved.
[0055] Add 5mL of perchloric acid to the dissolved sample, evaporate until fumes of perchloric acid are emitted, and cool slightly. Add 20mL of distilled water to dissolve the salt, dilute to the mark with water, and shake well to obtain solution a.
[0056] Accurately pipette 10.00mL test solution from solution a into a 100mL volumetric flask, add 7mL potassium sodium tartrate, 7mL ammonium citr...
Embodiment 2
[0063] Example 2 Weigh 0.5000 g of the sample (standard sample 1#), accurate to 0.0001 g.
[0064] The process of dissolving the sample is the same as in Example 1.
[0065] Add 5mL of perchloric acid to the dissolved sample, evaporate until fumes of perchloric acid are emitted, and cool slightly. Add 20mL of distilled water to dissolve the salt, dilute to the mark with water, and shake well to obtain solution a.
[0066] Accurately pipette 10.00mL test solution from solution a into a 100mL volumetric flask, add 10mL potassium sodium tartrate, 10mL ammonium citrate, 10mL sodium hydroxide, 2mL ammonium persulfate, 10mL dimethylglyoxime, dilute to the mark with water, mix uniform; wherein, the order of adding the masking agent is: first add sodium potassium tartrate, then ammonium citrate.
[0067] Place it for 10-20min, that is, after the chelation reaction is sufficient, use the reference solution as a reference, measure the color at 530nm on a spectrophotometer, and measure...
Embodiment 3
[0072] Example 3 Weigh 0.5000 g of the sample (standard sample 1#), accurate to 0.0001 g.
[0073] The process of dissolving the sample is the same as in Example 1.
[0074] Add 5mL of perchloric acid to the dissolved sample, evaporate until fumes of perchloric acid are emitted, and cool slightly. Add 20mL of distilled water to dissolve the salt, dilute to the mark with water, and shake well to obtain solution a.
[0075] Accurately pipette 10.00mL test solution from solution a into a 100mL volumetric flask, add 12mL sodium potassium tartrate, 12mL ammonium citrate, 10mL sodium hydroxide, 2mL ammonium persulfate, 10mL dimethylglyoxime, dilute to the mark with water, mix uniform; wherein, the order of adding the masking agent is: first add sodium potassium tartrate, then ammonium citrate.
[0076] Place it for 10-20min, that is, after the chelation reaction is sufficient, use the reference solution as a reference, measure the color at 530nm on a spectrophotometer, and measure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com