Preparation method of capecitabine
A capecitabine and flucytosine technology, applied in the field of drug preparation, can solve problems such as high price, achieve the effects of reducing production cost and consumption, solving environmental pollution, and being easy to control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
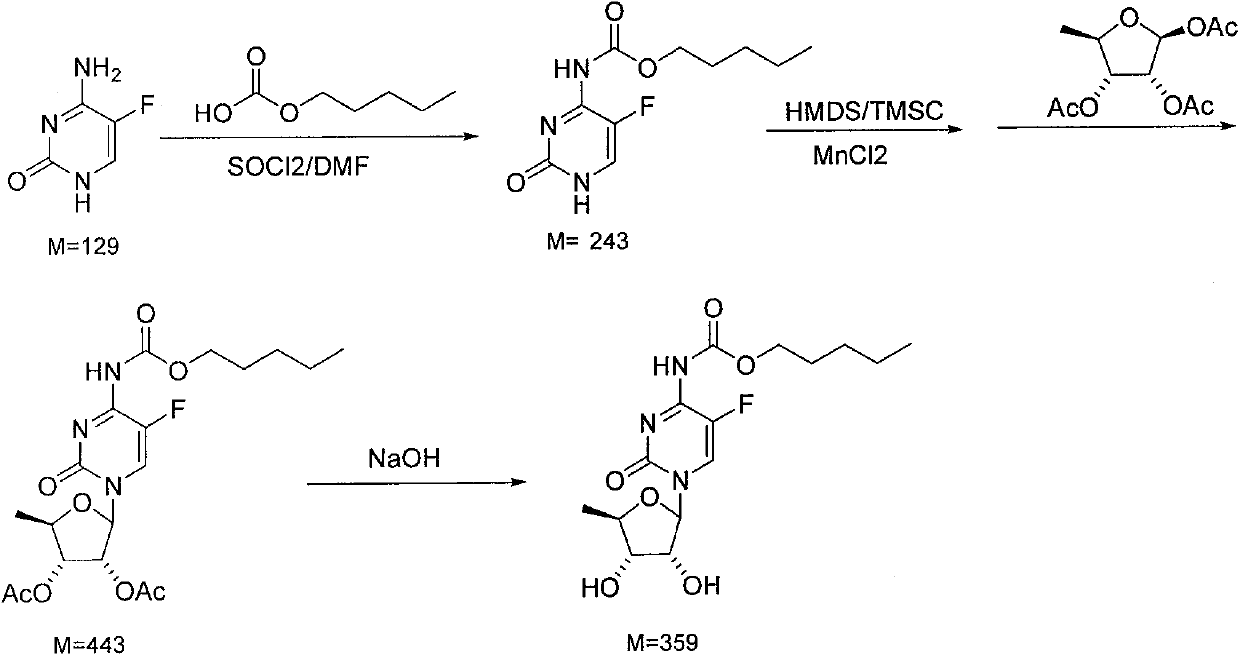
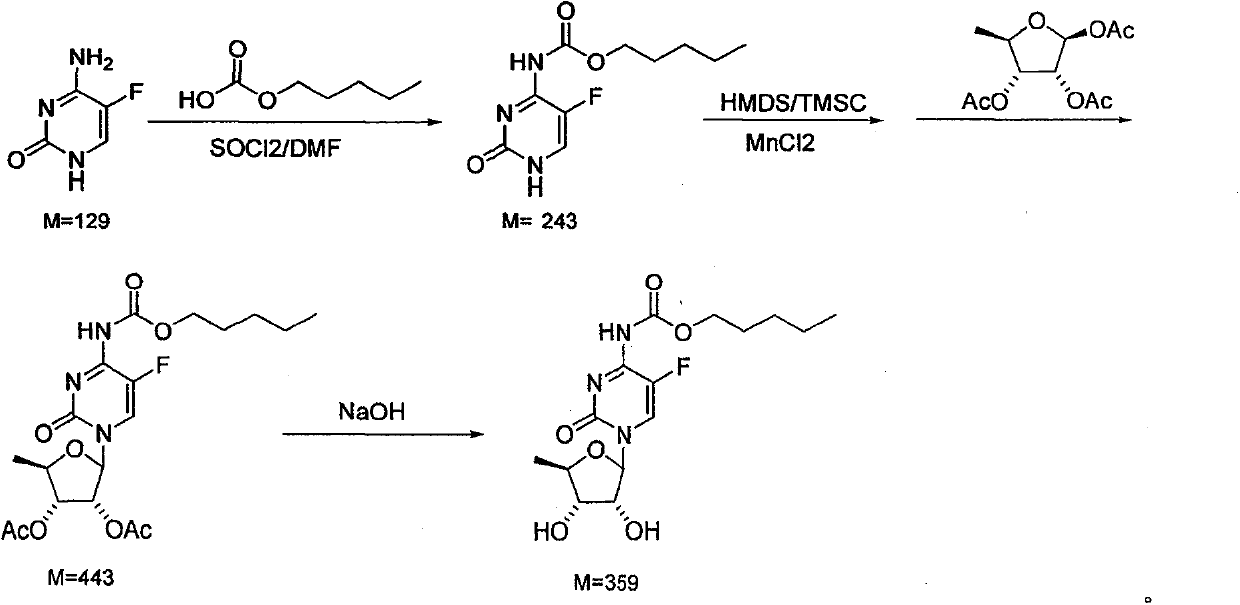
[0013] 1. Add 60ml (0.46mol) of n-pentyl carbonate, 5ml of DMF, and 60ml of dichloromethane to the reactor in turn, stir, cool to 0°C, and dropwise add 60.3g (0.51mol) of thionyl chloride + 60ml of dichloromethane to form solution, control the dropping temperature at -2-0°C, after dropping, keep warm for 2-3 hours. Add 20ml of water, stir to remove the water layer, add 38g (0.3mol) of 5-fluorocytosine and 30ml of triethylamine to the organic layer, stir and react at room temperature for 4 hours, add 30ml of water, stir to remove the water layer, organic layer Dry over anhydrous magnesium sulfate, remove the solvent, and obtain 79.4 g of a white solid product, which is directly used in the next step without refining;
[0014] 2. Stir 44.1g (0.35mol) of anhydrous manganese chloride, 67.6g (0.26mol) of 1,2,3-triacetyl-5-deoxy-D-ribose and 200ml of dichloromethane to form a solution, then cool to 0°C Prepare as follows; add 100ml of toluene, 15.9ml of hexamethyldisilazane, and 9....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com