Preparation method of clevidipine butyrate intermediate
A kind of technology of clevidipine butyrate and intermediate, applied in the direction of organic chemistry and the like, can solve the problems of long reaction steps, complicated operation, low yield and the like, and achieves the effects of mild reaction conditions, good hydrolysis selectivity and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
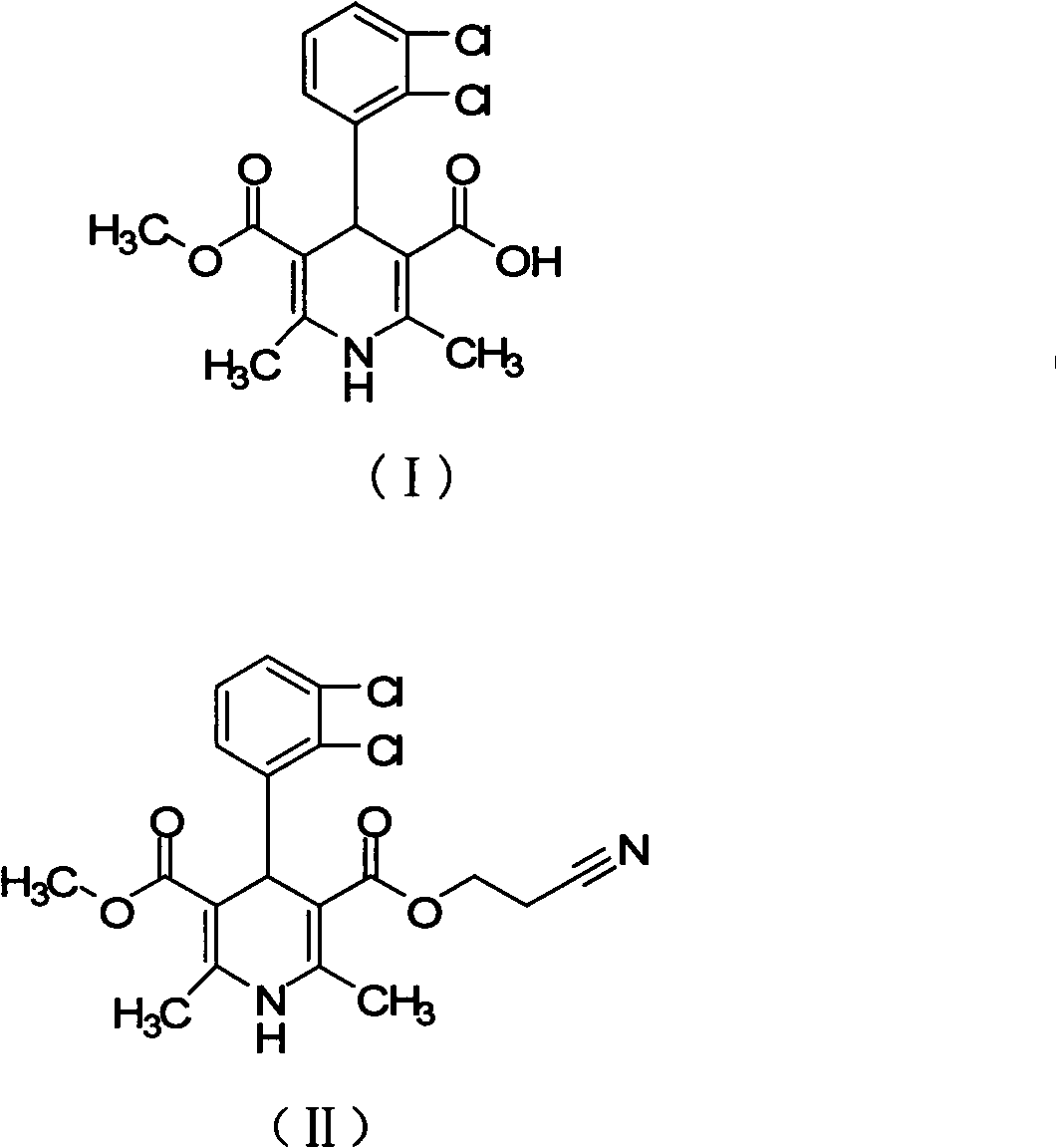
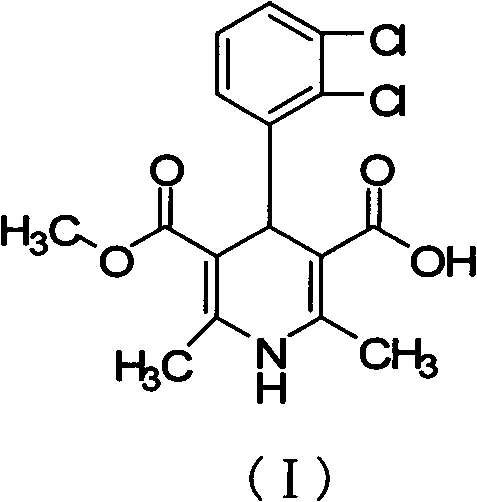
[0023] Methyl(1-cyanomethyl)methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate (II) Preparation of 3-(2,3-dichlorophenyl)-2-acetylbenzoic acid methyl ester (27.3 g, 0.1 mol), 3-amino-2-butenoic acid (2-cyano) ethyl ester (15.4 g, 0.1mol) was dissolved in methanol (340ml), stirred and refluxed for 7 hours, added activated carbon (8g) for decolorization for 1 hour, hot filtered, crystallized, filtered to obtain off-white or light yellow solid II (37.4g, 88.3%) , 1 HNMR (CDCH 3 ): 8.75 (brs, 1H, -NH), 7.05-7.60 (m, 3H, Ar-H), 4.78 (s, 1H, C 4 -H), 4.52(t, 2H, -OCH 2 -), 3.77(s, 3H, -COOCH 3 ), 2.69(t, 2H, -CH 2 CN) 2.26(s, 6H, 2×-CH 3 ).
Embodiment 2
[0025] Methyl(1-cyanomethyl)methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate (II) Preparation of 3-(2,3-dichlorophenyl)-2-acetylbenzoic acid methyl ester (27.3 g, 0.1 mol), 3-amino-2-butenoic acid (2-cyano) ethyl ester (15.4 g, 0.1mol) was dissolved in acetone (280ml), stirred and refluxed for 8 hours, added activated carbon (8g) for decolorization for 1 hour, heated, filtered, crystallized, and filtered to obtain off-white or light yellow solid II (29.1g, 68.6%)
Embodiment 3
[0027] Methyl(1-cyanomethyl)methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate (II) Preparation of 3-(2,3-dichlorophenyl)-2-acetylbenzoic acid methyl ester (27.3 g, 0.1 mol), 3-amino-2-butenoic acid (2-cyano) ethyl ester (15.4 g, 0.1mol) was dissolved in dichloromethane (520ml), stirred and refluxed for 7.5 hours, added activated carbon (8g) for decolorization for 1 hour, filtered hot, crystallized, and filtered to obtain off-white or light yellow solid II (22.3g, 52.7 %)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com