Manufacturing process of electrolyte for oxidation reduction cell
A manufacturing method and electrolyte technology, which are applied in indirect fuel cells, final product manufacturing, sustainable manufacturing/processing, etc., can solve the problems of complex manufacturing process, danger, delay in the popularization of redox batteries, etc., and achieve easy drug management and observation. , automatic operation simple and easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
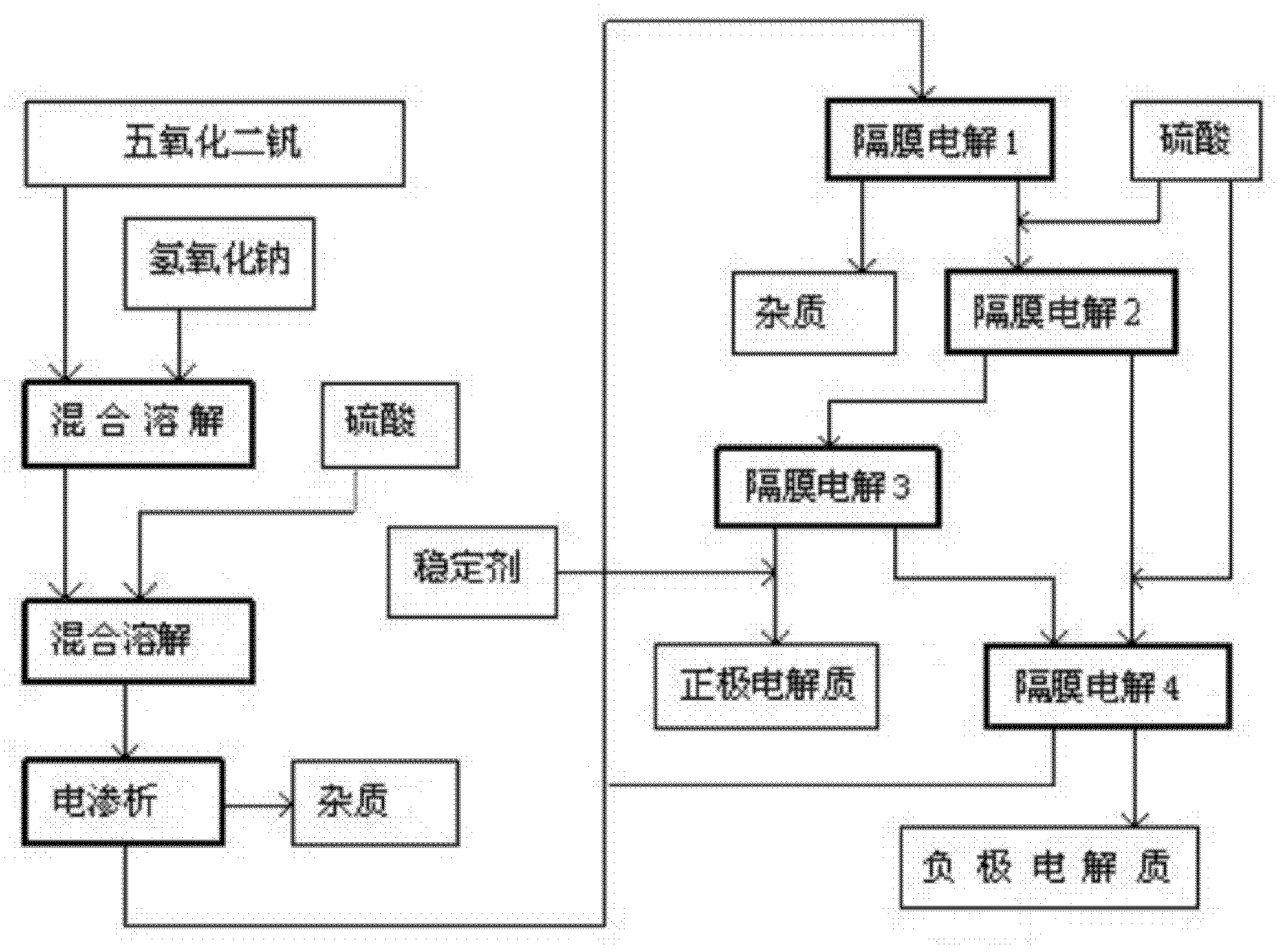
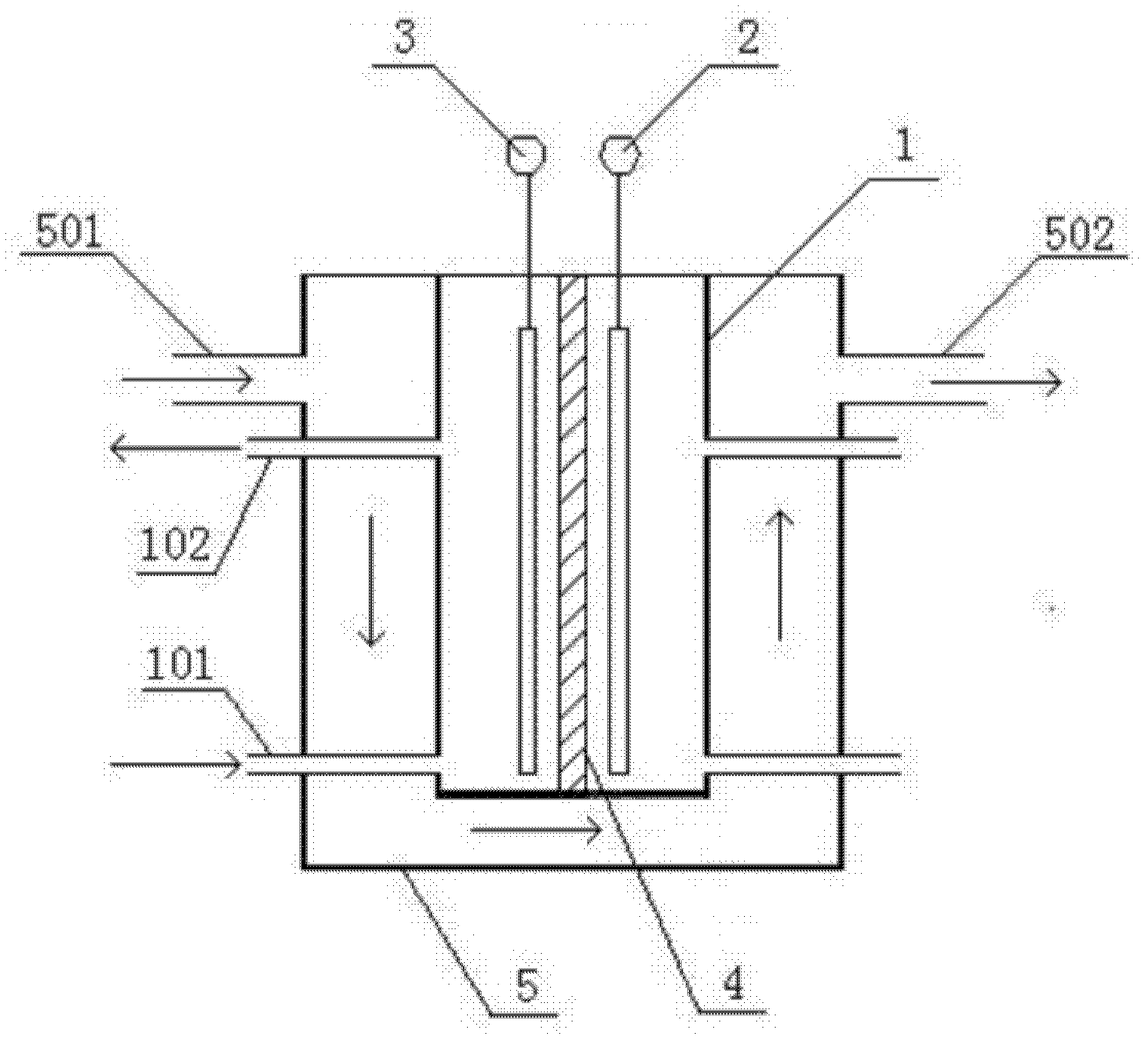
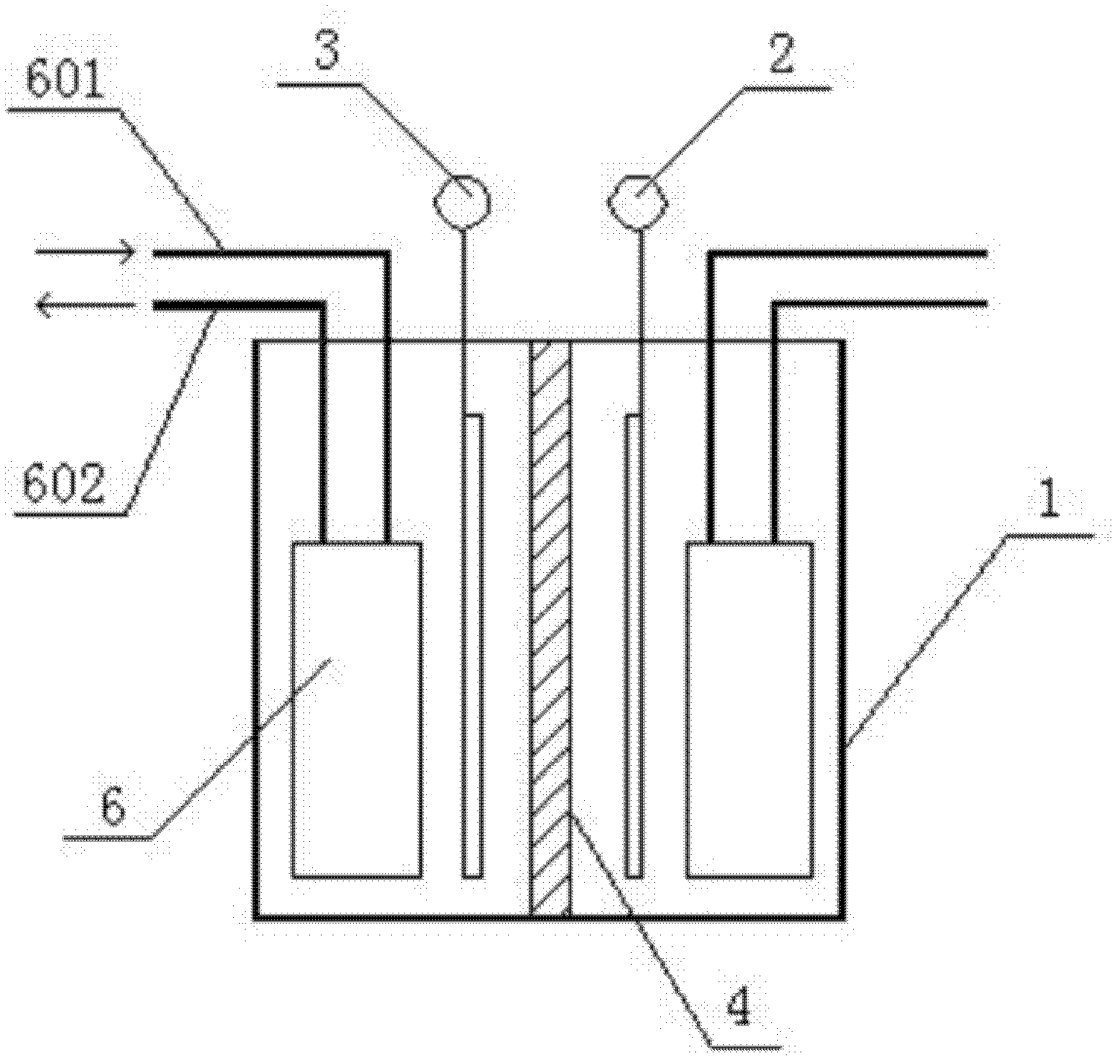
[0041] Such as figure 1 A method for manufacturing an electrolyte for a redox battery shown, the process steps are as follows:
[0042] (1) Slowly add vanadium pentoxide to the aqueous sodium hydroxide solution with a concentration of 20wt% and keep stirring, the stirring rate is controlled at 120rpm, and the addition of vanadium pentoxide is 25wt% of the quality of the alkaline aqueous solution. The speed of adding vanadium pentoxide is preferably not to produce a large amount of precipitates, and the adding time is 30 minutes;
[0043] (2) After the vanadium pentoxide addition is completed, continue to stir, and the stirring rate is controlled at 30rpm, and continues to stir for 50 minutes to make it fully dissolve;
[0044] (3) Slowly add sulfuric acid with a concentration of 25 wt% into the above system to react to generate vanadyl(IV) sulfate, stop adding when the pH value of the system reaches 2.0 to 2.2, and continue the reaction for 30 minutes;
[0045] (4) remove so...
Embodiment 2
[0052] Such as figure 1 A method for manufacturing an electrolyte for a redox battery shown, the process steps are as follows:
[0053] (1) Slowly add vanadium pentoxide to the aqueous sodium hydroxide solution with a concentration of 30wt% and keep stirring, the stirring rate is controlled at 80rpm, and the addition of vanadium pentoxide is 40wt% of the quality of the alkaline aqueous solution. The speed of adding vanadium pentoxide is advisable not to produce a large amount of precipitates, and the adding time is 60 minutes;
[0054](2) After the vanadium pentoxide addition is completed, continue to stir, and the stirring rate is controlled at 50rpm, and continues to stir for 30 minutes to make it fully dissolve;
[0055] (3) Slowly add sulfuric acid with a concentration of 20 wt% into the above system to react to generate vanadyl(IV) sulfate, stop adding when the pH value of the system reaches 2.0 to 2.2, and continue the reaction for 20 minutes;
[0056] (4) remove sodiu...
Embodiment 3
[0062] Such as figure 1 A method for manufacturing an electrolyte for a redox battery shown, the process steps are as follows:
[0063] (1) Slowly add vanadium pentoxide to the potassium hydroxide aqueous solution with a concentration of 20wt% and keep stirring, the stirring rate is controlled at 150rpm, and the addition of vanadium pentoxide is 25wt% of the potassium hydroxide aqueous solution quality. The speed of adding vanadium pentoxide is preferably not to produce a large amount of precipitates, and the adding time is 30 minutes;
[0064] (2) After the vanadium pentoxide addition is completed, continue to stir, and the stirring rate is controlled at 60rpm, and continues to stir for 30 minutes to make it fully dissolve;
[0065] (3) Slowly add sulfuric acid with a concentration of 30wt% into the above system to react to generate vanadyl(IV) sulfate, stop adding when the pH value of the system reaches 2.0 to 2.2, and continue the reaction for 30 minutes;
[0066] (4) rem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com