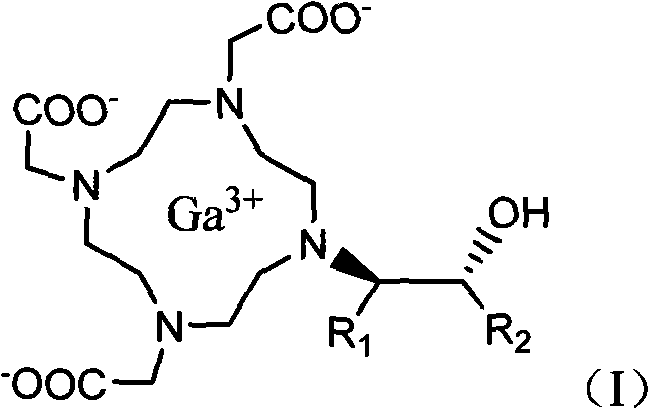
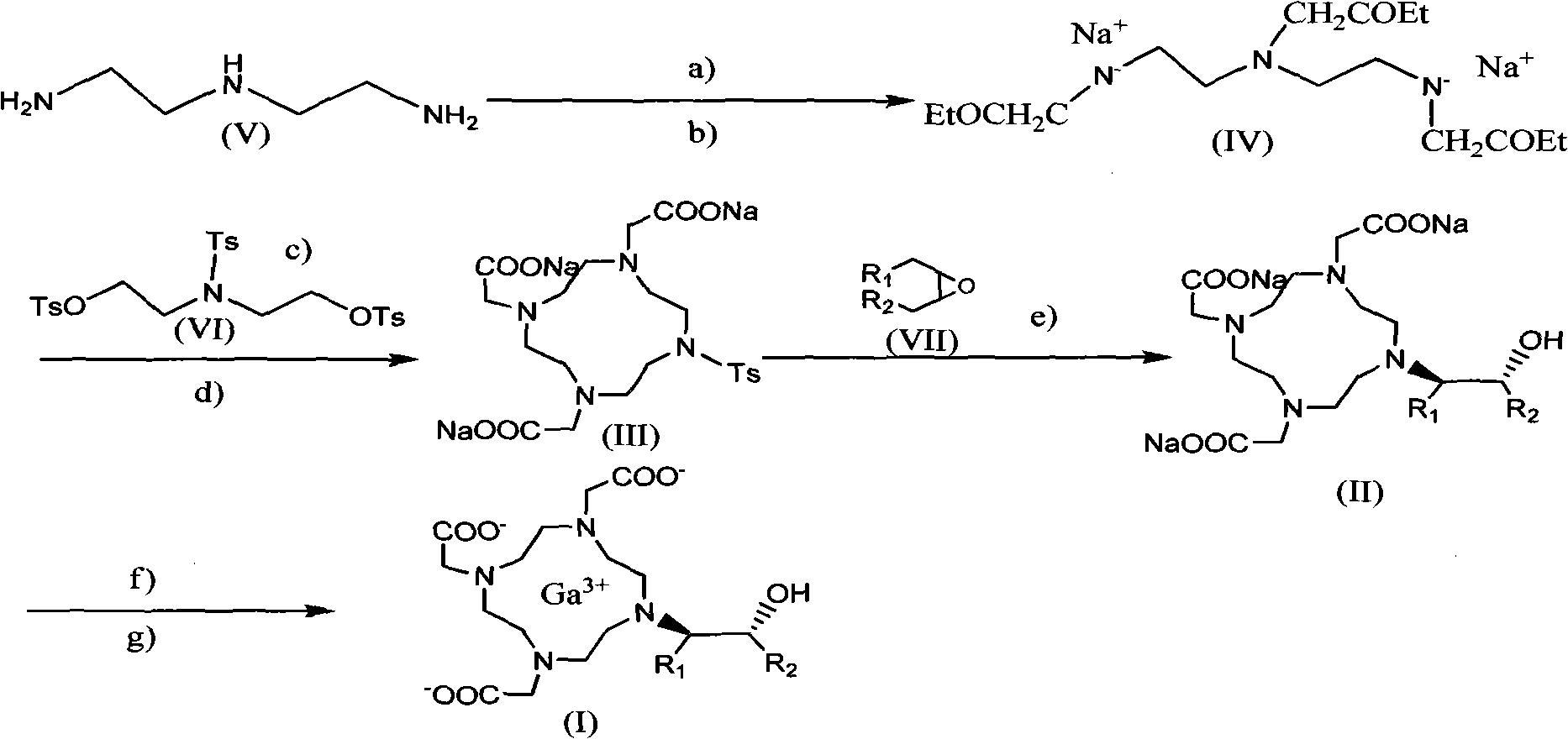
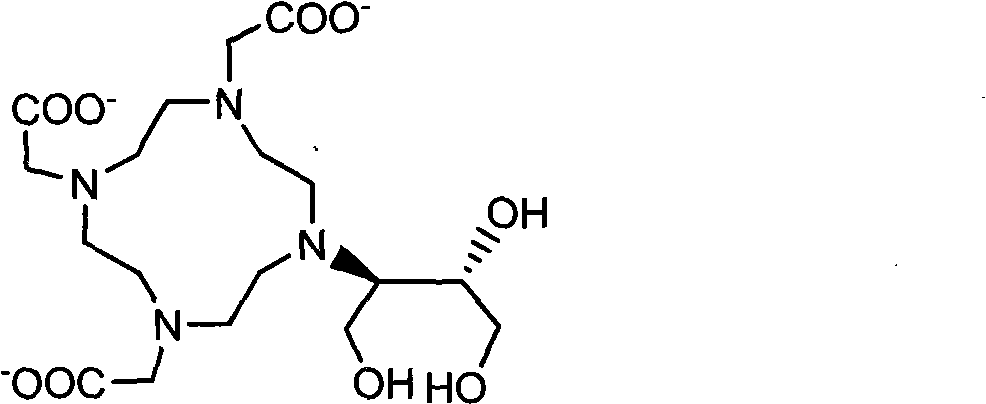
Preparation method of magnetic resonance imaging contrast agent
A technology of magnetic resonance imaging and contrast agent, applied in the direction of nuclear magnetic resonance/magnetic resonance imaging contrast agent, organic chemistry, etc., can solve the problems of unsuitability for industrial production, high cost of industrial production, low total yield, etc. The effect of low equipment requirements and a wide range of sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: Preparation of gadobutrol
[0026]
[0027] A) Preparation of N, N', N"-tris(2-ethoxy-2-carbonylethyl)diethylenetriamine
[0028] Disperse 1.03kg (10mol) of diethylenetriamine and 100g of anhydrous potassium carbonate in 3L of acetonitrile, slowly add 3.43kg (28mol) of ethyl chloroacetate dropwise while stirring, and control the dropping time for not less than 3 hours. After the addition was complete, the reaction was stirred for 3 hours, and the acetonitrile was recovered under reduced pressure to obtain the desired compound.
[0029] B) Preparation of N, N', N"-tris(2-ethoxy-2-carbonylethyl) diethylenetriamine disodium salt (IV)
[0030] Dissolve and disperse the product in A) in 1.5L of ethanol, add 1.36kg (20mol) of sodium ethoxide, heat and stir under reflux for 5 hours, and filter to obtain N,N',N"-tris(2-ethoxy- 2-Carbonylethyl) diethylenetriamine disodium salt 2.93 kg (8.2 mol, yield 82%) of white solid, the purity was detected by HPLC, and th...
Embodiment 2
[0039] Embodiment 2: Synthetic gadolinium
[0040]
[0041] A)-D) steps are consistent with the synthesis method of gadobutrol in Example 1.
[0042]E) Dissolve the target product obtained in D) in water, adjust the pH to 12.3 with hydrochloric acid, add 363 g (6.26 mol) of propylene oxide, react at 40 ° C for 4 hours and react at 80 ° C for 8 hours, and then cool the solution to 50°C, add 6.2mol gadolinium trichloride in 5.5kg aqueous solution. After 1 hour the mixture was cooled to 17°C and acidified to pH 1.7 with concentrated hydrochloric acid and kept stirring for 2 hours. Then the temperature was raised to 50° C., the pH was adjusted to 7 with sodium hydroxide, and the reaction was stirred for 1 hour.
[0043] The desalination and purification of gadolidol were the same as in Example 1F), and finally 2.49kg of white powder was obtained with a total yield of 55%. IR, MS and NMR were consistent with the specified structure, and its purity was 99.1% by HPLC analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com