High-temperature stable oxygen-carrier-containing pharmaceutical composition
A technology of high temperature stability and composition, applied in the directions of drug combination, pharmaceutical formulation, chemical instrument and method, etc., can solve the problem of unacceptable high percentage of dimer units, vasoconstriction, and unsatisfactory administration of hemoglobin composition to breastfeeding animals, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
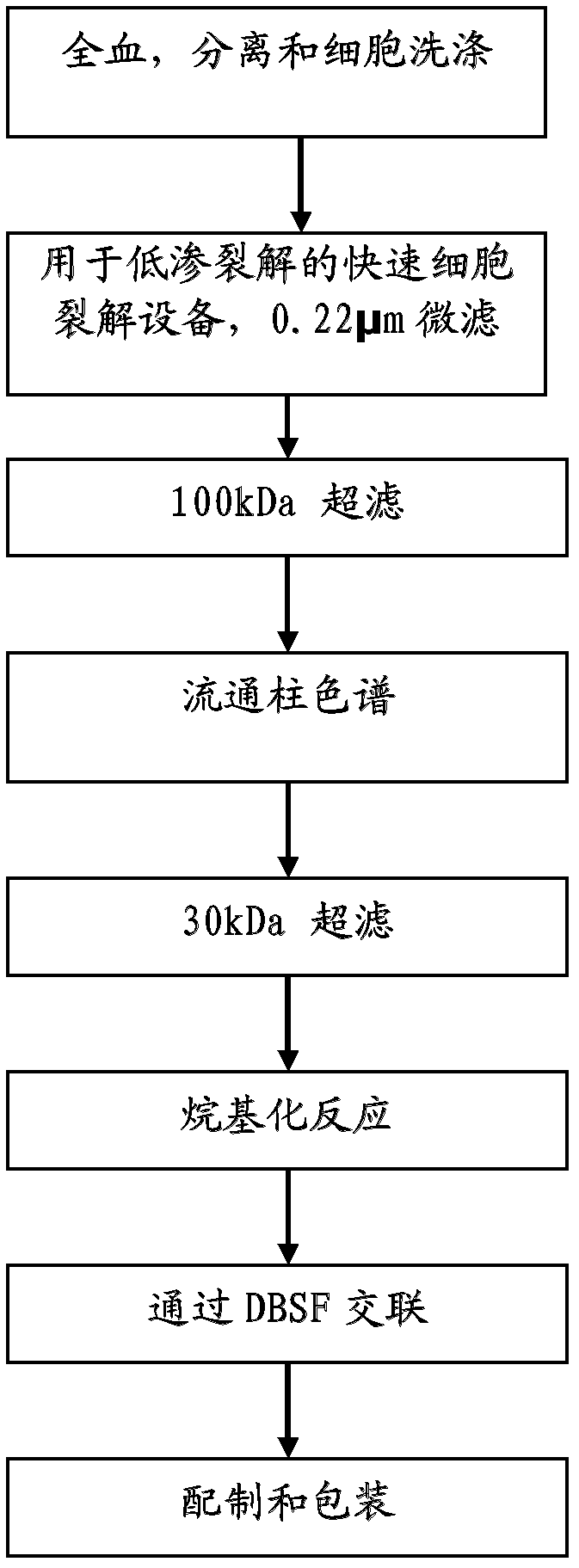
[0067] total process
[0068] A schematic flow diagram of the method of the present invention is shown in figure 2 middle. Bovine whole blood was collected in closed sterile containers / bags containing 3.8% (w / v) sodium citrate solution as anticoagulant. The blood is then immediately mixed well with a sodium citrate solution to inhibit clots. Red blood cells (RBCs) are separated and collected from plasma and other smaller blood cells by an apheresis mechanism. A "cell washer" was used with a gamma sterilized disposable centrifuge bowl for this step. RBCs were washed with an equal volume of 0.9% (w / v sodium chloride) saline.
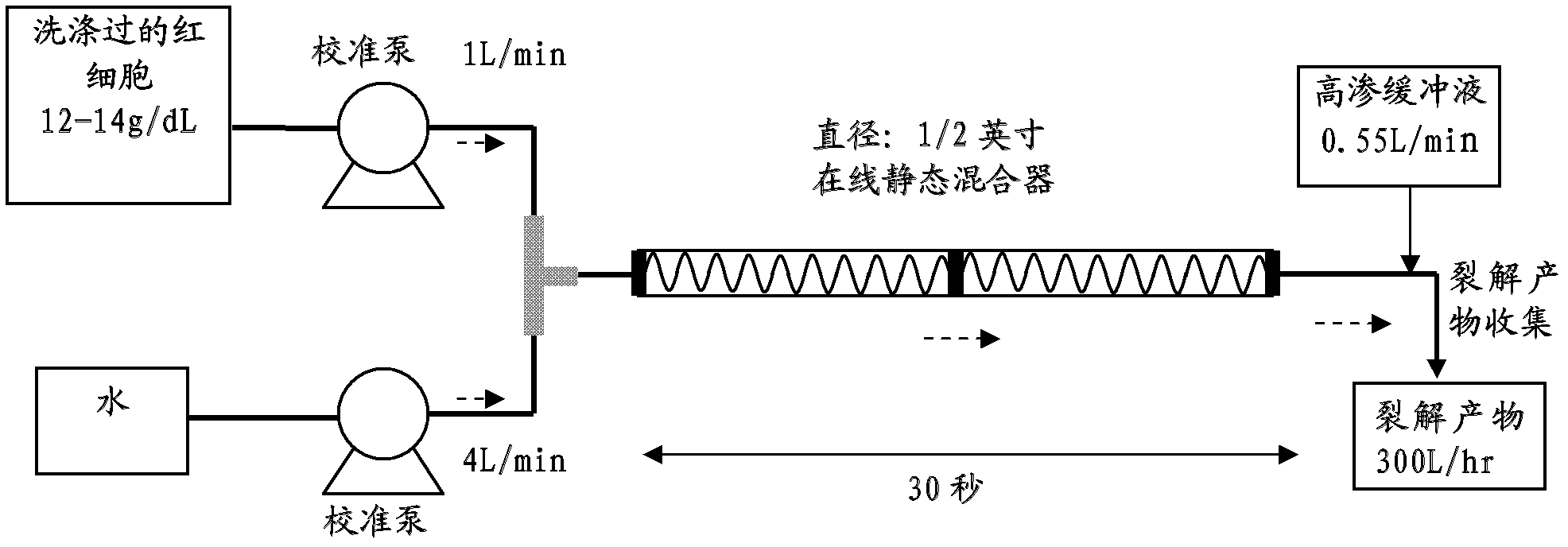
[0069] Hypotonic shock to the cell membrane of red blood cells to lyse washed red blood cells to release the hemoglobin contents. image 3 An RBC-specific rapid cell lysis device was drawn for this purpose. After RBC lysis, hemoglobin molecules were separated from other proteins by tangential flow ultrafiltration using a 100 kDa membrane. Hemoglobi...
Embodiment 2
[0072] Timed & Controlled Hypotonic Lysis and Filtration
[0073] Whole bovine blood is collected fresh and shipped refrigerated. Red blood cells were separated from plasma by a cell washer followed by 0.65 μm filtration. After washing the red blood cell (RBC) filtrate with 0.9% saline, the filtrate was disrupted by hypotonic lysis. by using image 3 The rapid cell lysis device drawn in the paper for hypotonic lysis. The rapid cell lysis device includes a static mixer to aid in cell lysis. An RBC suspension containing a controlled concentration of hemoglobin (in the range of 12-14 g / dL) was mixed with 4 volumes of purified water to hypotonically impact the RBC cell membrane. The timing of the hypotonic shock is controlled to avoid undesired lysis of leukocytes and platelets. The hypotonic solution was passed through the static mixer section of the rapid cell lysis device for about 30 seconds. The shock was terminated after 30 seconds as the lysate exited the static mixer...
Embodiment 3
[0076] Virus clearance studies on stroma-free hemoglobin solutions
[0077] In order to demonstrate the safety of the product described in the present invention, we demonstrated the virus clearance capacity of (1) 0.65 μm diafiltration step and (2) 100 kDa ultrafiltration step through virus validation studies. We implemented both methods with scaled-down small-scale reaction systems to which different model viruses were artificially added, such as encephalomyocarditis virus, pseudorabies virus, bovine viral diarrhea virus (bovine viral diarrhea virus) and bovine parvovirus (bovine parvovirus). Four types of viruses were used in this study, see Table 2 below. These viruses differ in their biophysical and structural characteristics and in their resistance to physical and chemical agents or treatments.
[0078] Table 2
[0079]
[0080] The virus validation scheme is briefly shown in Table 3 below.
[0081] table 3
[0082]
[0083] Table 4 below summarizes the logarit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com