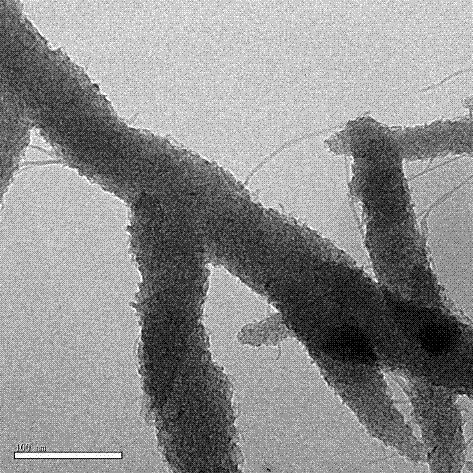
Polyaniline nanofiber/manganese dioxide nanorod composite material and preparation method thereof
A nanofiber, manganese dioxide technology, applied in the field of chemistry, can solve rare problems and achieve the effects of high product reproducibility, high stability and saving experimental reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: Preparation of polyaniline nanofiber / manganese dioxide nanorod composite material
[0031] (1) Raw material preparation: Aniline was distilled under reduced pressure before use, and stored in a 4°C refrigerator for later use. Potassium permanganate was prepared into a 0.10 mol / L aqueous solution with deionized water before use. Other chemical reagents were of analytical grade, and water was deionized water.
[0032] (2) Preparation of polyaniline nanofibers: Dissolve 0.30 ml aniline in 10 ml 1.0 mol / L HCl aqueous solution, and add 10 ml ammonium persulfate aqueous solution (0.18 g ammonium persulfate, 1.0 mol / L HCl aqueous solution) to it at one time , fully stirred at 25 °C for half a minute, and then reacted under static conditions for 6 hours, and the obtained polyaniline nanofibers had a particle size of 40-60 nm. Centrifuge the reaction system containing the precipitate to obtain a precipitate, then centrifuge and wash it with deionized water for mo...
Embodiment 2
[0035] Embodiment 2: Preparation of polyaniline nanofiber / manganese dioxide nanorod composite material
[0036] (1) Raw material preparation: Aniline was distilled under reduced pressure before use, and stored in a 4°C refrigerator for later use. Potassium permanganate was prepared into a 0.10 mol / L aqueous solution with deionized water before use. Other chemical reagents were of analytical grade, and water was deionized water.
[0037] (2) Preparation of polyaniline nanofibers: Dissolve 0.30 ml aniline in 10 ml 1.0 mol / L HCl aqueous solution, and add 10 ml ammonium persulfate aqueous solution (0.18 g ammonium persulfate, 1.0 mol / L HCl aqueous solution) to it at one time , fully stirred at 25°C for half a minute, and then reacted under static conditions for 6 hours, and the obtained polyaniline nanofibers had a particle size of 40-60 nm. The reaction system containing the precipitate was centrifuged to obtain a precipitate, and then centrifuged and washed with deionized wate...
Embodiment 3
[0040] Embodiment 3; Preparation of polyaniline nanofiber / manganese dioxide nanorod composite material
[0041] (1) Raw material preparation: Aniline was distilled under reduced pressure before use, and stored in a 4°C refrigerator for later use. Potassium permanganate was prepared into a 0.01 mol / L aqueous solution with deionized water before use. Other chemical reagents were of analytical grade, and water was deionized water.
[0042](2) Preparation of polyaniline nanofibers: Dissolve 0.30 ml aniline in 10 ml 1.0 mol / L HCl aqueous solution, and add 10 ml ammonium persulfate aqueous solution (0.18 g ammonium persulfate, 1.0 mol / L HCl aqueous solution) to it at one time , fully stirred at 25 °C for half a minute, and then reacted under static conditions for 6 hours, and the obtained polyaniline nanofibers had a particle size of 40-60 nm. The reaction system containing the precipitate was centrifuged to obtain a precipitate, and then centrifuged and washed with deionized wate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com