Medicine for eliminating breast cancer cells with high efficiency and high specificity
A breast cancer cell and specific technology, applied in the field of medicine and biology, can solve the problem of not being able to kill breast cancer stem cells, and achieve the effect of no liver and kidney toxicity, exact drug effect, and low toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
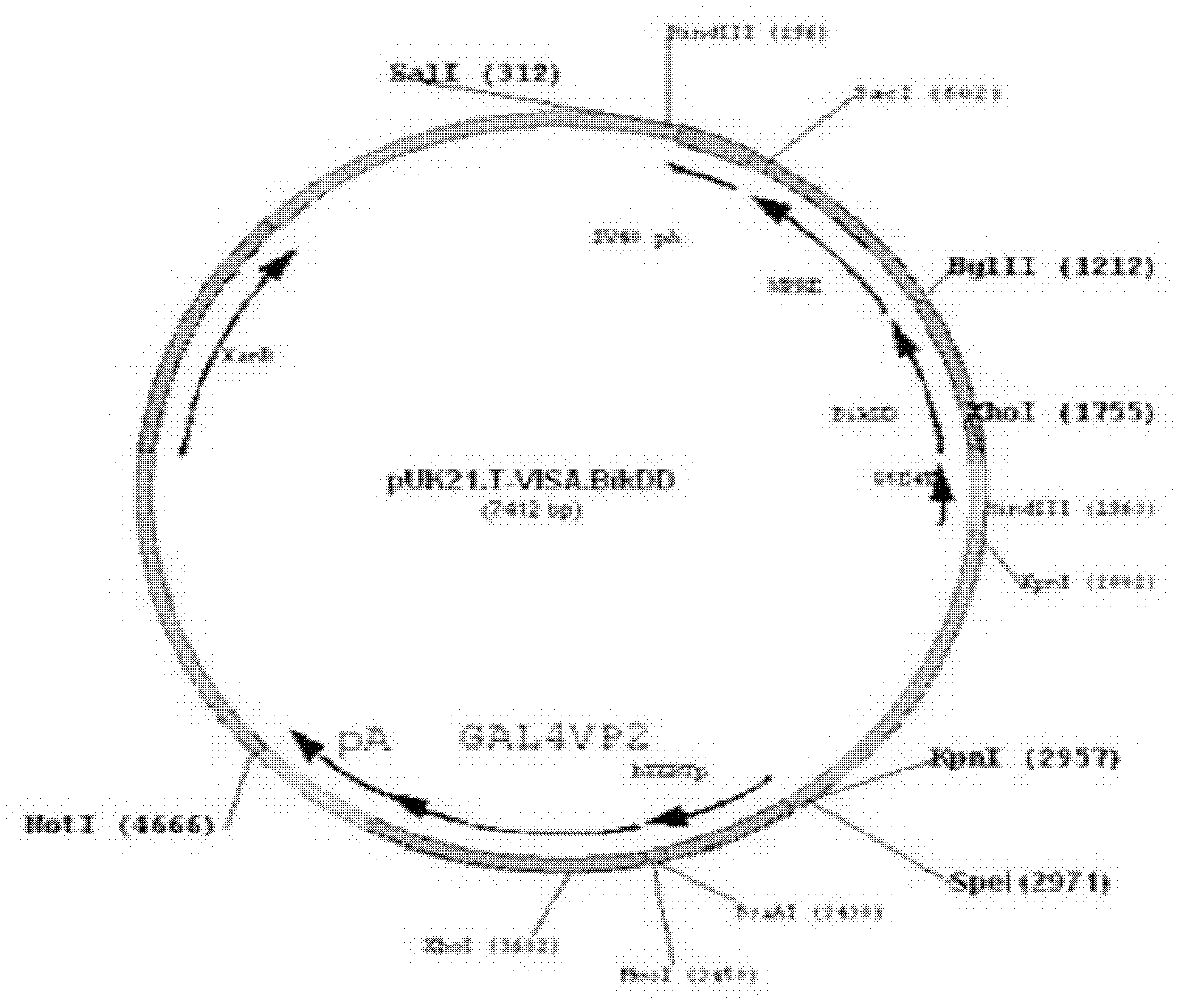
[0017] Example 1: Construction of T-VISA-BikDD therapeutic vector
[0018] (1) pCRII-TOPO-hTERT plasmid clone
[0019] 1. The DNA of breast cancer MCF-7 cells (purchased from ATCC) was extracted by conventional methods.
[0020] 2. Using the DNA as a template, design PCR primers for amplifying hTERT, hTERT PCR upstream (Forward) and downstream (Reverse) primers:
[0021] Forward: 5′-ata tct aga ggc ccc tcc ctc ggg tta ccc cac agc-3′(XbaI)
[0022] Reverse: 5'-ata gat ctt atg cgg ccg ccc acg tgc gca gca gga cgc agc gc-3'.
[0023] 3. Using breast cancer MCF-7 cell DNA as template, hTERT PCR primers (Forward: 5′-ata tct aga ggc ccc tcc ctc ggg tta ccc cac agc-3′; Reverse: 5′-ata gat ctt atg cgg ccg ccc acg tgc gca gca gga cgc agc gc-3′), Pfu DNA polymerase, dNTP and PCR reaction solution, amplify to obtain the hTERT promoter (-416 to+1) product, the sequence of which is shown in SEQ ID NO.2. The PCR reaction system: 10×PCR buffer 5μl, upstream (Forward) and downstream (Reverse) primers e...
Embodiment 2
[0079] Example 2: Preparation of liposomes:
[0080] Remove the lipid from the refrigerator (store DOTAP at -20°C, and store cholesterol at -4°C) and return to room temperature. Heat two rotary evaporators in a water bath to 30°C and 50°C respectively. Weigh 68.75mg of cholesterol and put it into a 1000ml round bottom flask. Add 100 mg DOTAP and 25 mg Choroform to the round bottom flask. Turn the flask to mix well. Rotate the round-bottom flask in a water bath at 30°C for 2 minutes to make it evenly mixed and form a film on the wall of the flask. Turn on the vacuum aspirator and leave it at 30°C for 30 minutes. Add 8.9ml of preheated 5% glucose solution to dissolve the dried film, and rotate it quickly at 105 rpm for 45 minutes at 50°C. Then reduce the temperature to 35°C and rotate for 10 minutes. Seal the flask with plastic wrap (or paraffin) and leave it at room temperature overnight to avoid light. Measure the volume and add double distilled water to 8.9ml. The flask ...
Embodiment 3
[0081] Example 3: Preparation of T-VISA-BiKDD liposomes
[0082] The T-VISA-BikDD treatment carrier was dissolved in a 5% glucose solution to make the final concentration 1ug / ul. At the same time, the liposomes of Example 2 at the storage concentration (20mM) were diluted with 5% glucose solution to the working concentration (8mM) . 1μg DNA / ul T-VISA-BikDD treatment carrier was slowly added to 8mM liposomes and allowed to stand at room temperature for 20 minutes to react to form T-VISA-BikDD liposomes.
[0083] Then check the quality control such as verification, biological characteristics test and endotoxin, aseptic packaging. The finished product is tested, packaged, and stored at 4-8°C; put it at room temperature for 20 minutes before use. It can be directly used for in vitro research or in vivo research, or it can be diluted with 5% glucose solution for use.
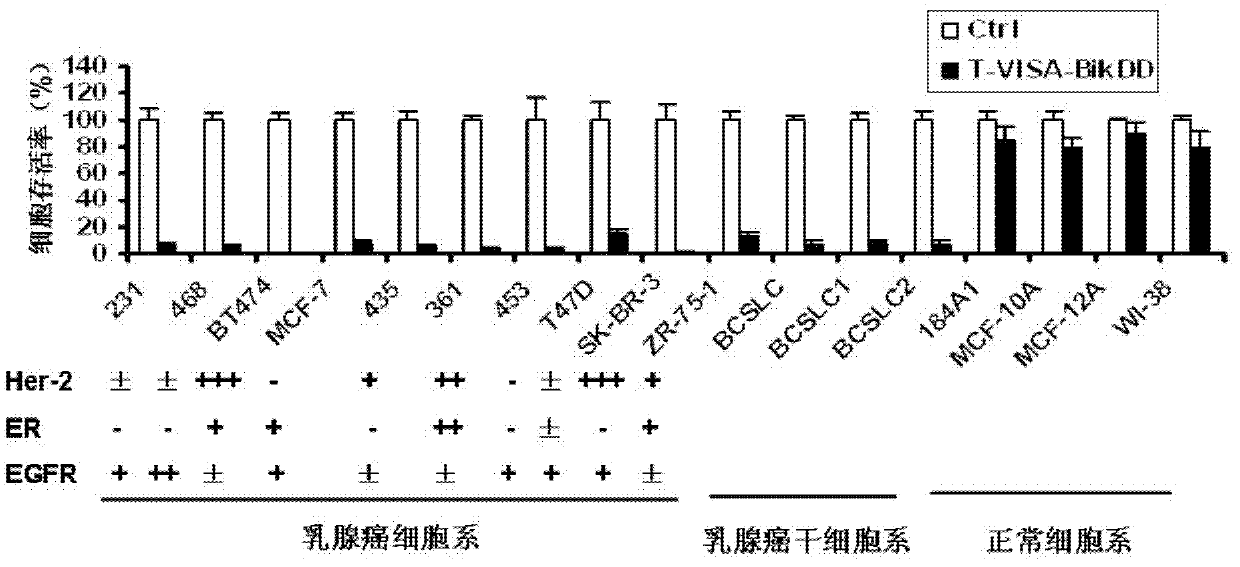
[0084] 2. Effect experiment:
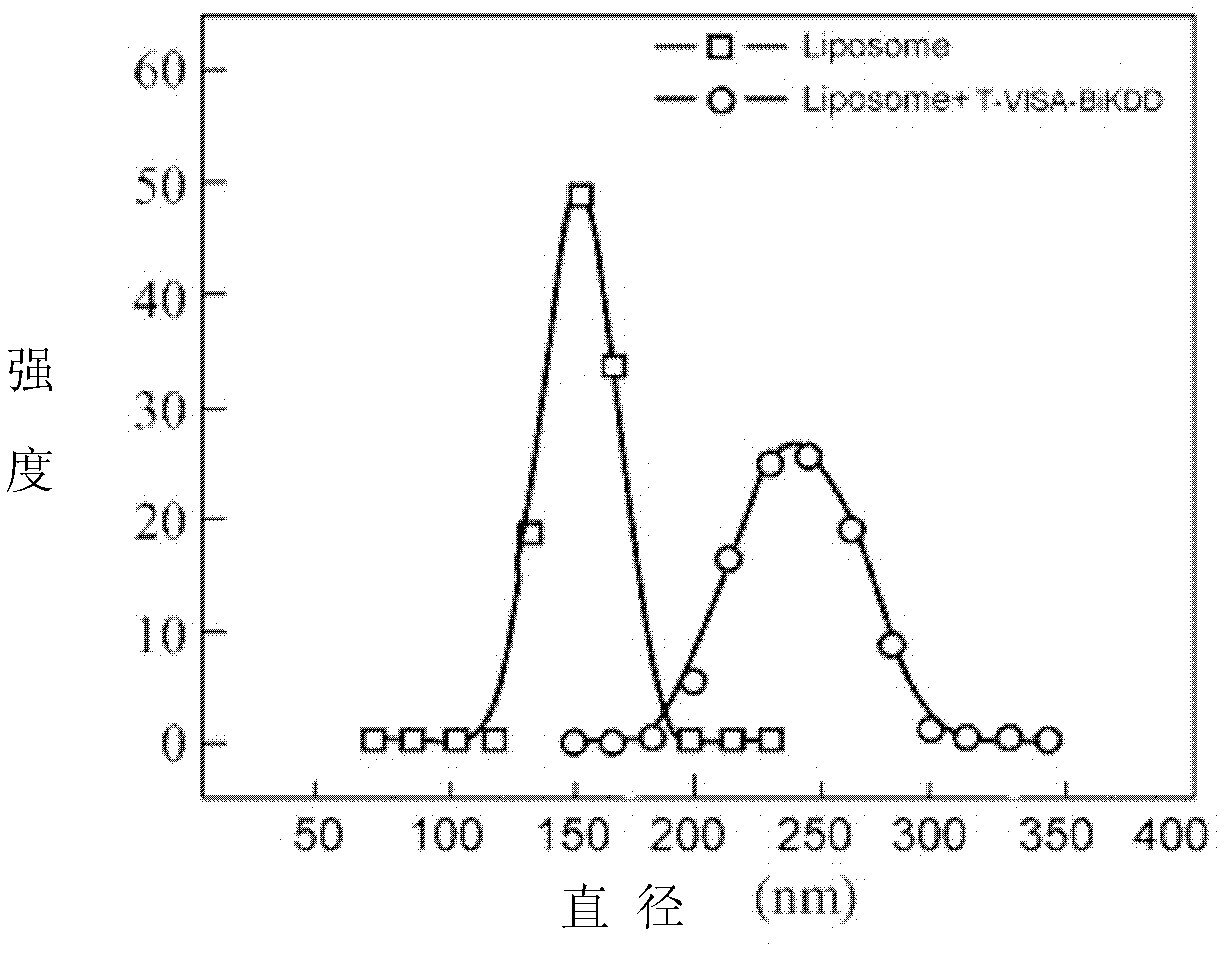
[0085] 1. Biological characteristics of T-VISA-BiKDD liposomes:
[0086] The biological ch...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com