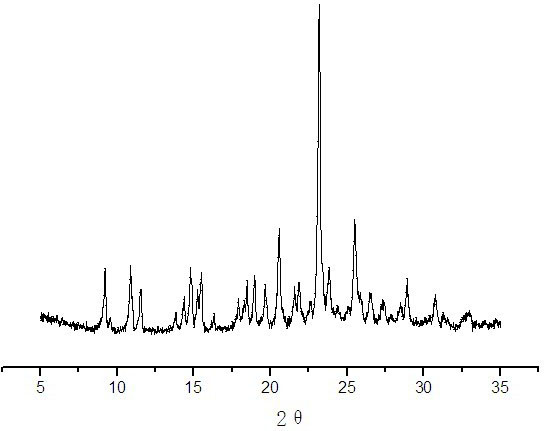
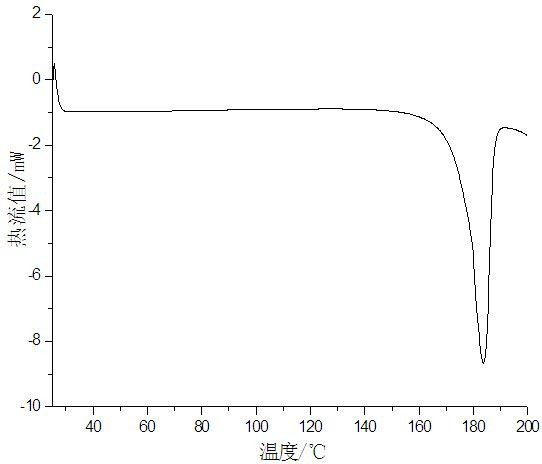
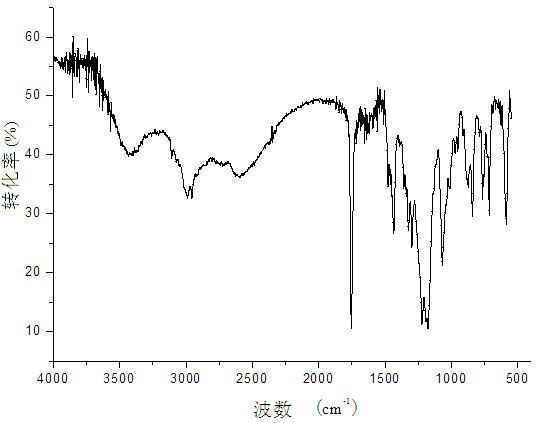
Crystallization method for preparing high-purity I-type clopidogrel hydrogen sulfate
A technology of clopidogrel hydrogen sulfate and clopidogrel free base, which is applied in the preparation of type I clopidogrel hydrogen sulfate and the field of preparation of type I clopidogrel hydrogen sulfate, which can solve the problem of short storage time and product crystal form Problems such as low purity and complex preparation process of type I clopidogrel bisulfate have achieved the effect of easy industrial production and high crystal purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Under the protection of nitrogen, add 5 grams of clopidogrel bisulfate type II, 50 milliliters of dichloromethane and 50 milliliters of water into a 250 milliliter three-neck flask, stir, cool down to 0~5 °C, and dropwise add 30 milliliters of 10% sodium carbonate aqueous solution. React until the pH value of the aqueous phase of the solution reaches 7-8, let stand to separate the layers, separate the organic phase, and extract the aqueous phase once with 50 ml of dichloromethane. The organic phases were combined and evaporated to dryness of dichloromethane to obtain 3.5 g of clopidogrel free base. Add 94.5 ml of ethyl acetate to the free base (ρ=0.9 g / cm 3 ), stirred to dissolve the free base completely, and filtered to obtain a clear solution. Control the temperature of the solution at 23°C, add 0.05 g of clopidogrel hydrogensulfate type I to the solution, mix well, and start to add 10.5 ml of 10% sulfuric acid ethyl acetate solution dropwise (control the amount of ...
Embodiment 2
[0034] Under nitrogen protection, add 6.5 g of clopidogrel camphorsulfonate, 50 ml of dichloromethane and 50 ml of water into a 250 ml three-neck flask, stir, cool down to 0~5°C, and dropwise add 30 ml of 10% sodium carbonate aqueous solution . React until the pH value of the aqueous phase of the solution reaches 7-8, let stand to separate the layers, separate the organic phase, and extract the aqueous phase once with 50 ml of dichloromethane. The organic phases were combined and evaporated to dryness of dichloromethane to obtain 3.6 g of clopidogrel free base. Add 97.2 ml of ethyl acetate to the free base (ρ=0.9g / cm 3 ), stirred to dissolve the free base completely, and filtered to obtain a clear solution. Control the temperature of the solution at 23°C, add 0.05 g of clopidogrel type I bisulfate to the solution, mix well, and start to add 10.8 ml of 10% ethyl sulfate acetate solution dropwise, and control the temperature of the solution at 23~25°C during the dropwise addit...
Embodiment 3
[0036]Under the protection of nitrogen, 4.8 grams of clopidogrel hydrobromide, 50 milliliters of dichloromethane and 50 milliliters of water were added to a 250 milliliter three-neck flask, stirred, cooled to 0~5 °C, and 30 milliliters of 10% sodium carbonate was added dropwise aqueous solution. React until the pH value of the aqueous phase of the solution reaches 7-8, let stand to separate the layers, separate the organic phase, and extract the aqueous phase once with 50 ml of dichloromethane. The organic phases were combined and evaporated to dryness of dichloromethane to obtain 3.4 g of clopidogrel free base. Add 91.8 ml of ethyl acetate to the free base (ρ=0.9 g / cm 3 ), stirred to dissolve the free base completely, and filtered to obtain a clear solution. Control the temperature of the solution at 23°C, add 0.05 g of clopidogrel type I bisulfate to the solution, mix well, and start to add 10.2 ml of 10% ethyl sulfate acetate solution dropwise, and control the temperature...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com