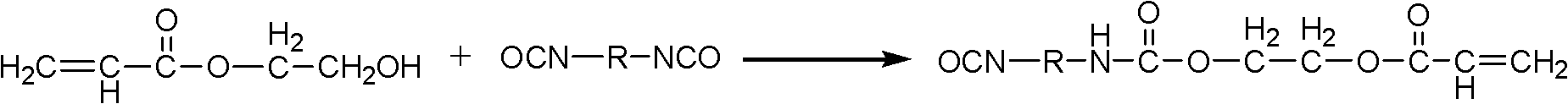Sulfonated aqueous UV polyurethane modified epoxy acrylate emulsion and its preparation method
A polyurethane modification, epoxy acrylic technology, applied in epoxy resin coatings, coatings, etc., can solve the problems of unstable storage, weak carboxyl electronegativity, poor oligomer hydrophilicity, etc., and achieve less side reactions. , The effect of flexible synthesis route and simple synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) Under the protection of nitrogen, add hydroxyethyl acrylate HEA or hydroxypropyl acrylate HPA and dibutyltin dilaurate (DBTDL) dropwise to isophorone diisocyanate (IPDI), keep the reaction temperature at 40°C, monitor The concentration of -NCO in the system changes until the concentration of -NCO no longer changes, the molar ratio of HEA or HPA to diisocyanate is 1:1; the mass ratio of dibutyltin dilaurate to hydroxyethyl acrylate or hydroxypropyl acrylate is 0.9 : 100, to obtain the semi-adduct of diisocyanate;
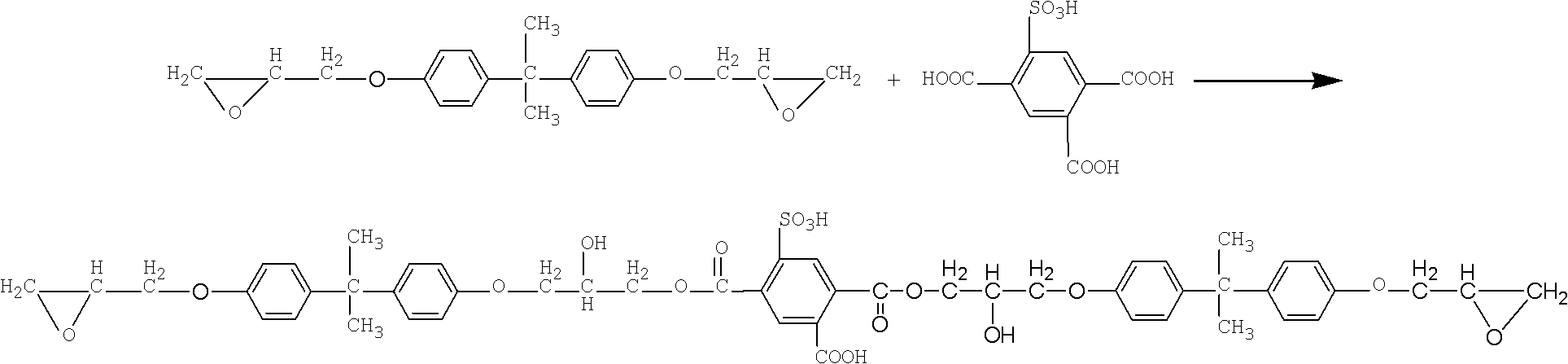
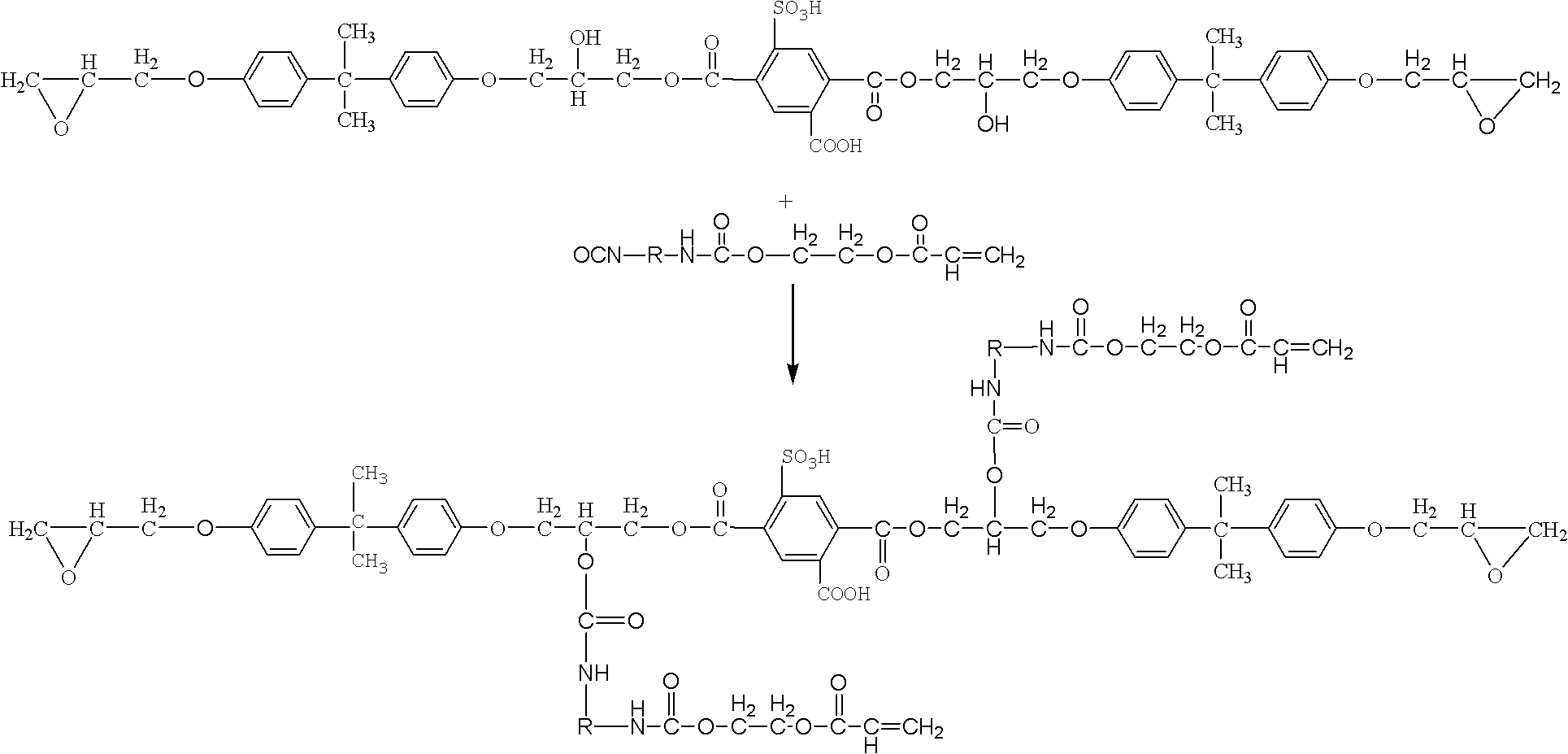
[0044] (2) The ring-opening esterification reaction of epoxy resin E51 and tricarboxybenzenesulfonic acid under the effect of catalyst triphenylphosphine, the mol ratio of epoxy resin and tricarboxybenzenesulfonic acid is 2: 1, and the initial acid value is 208.8mgKOH / g resin, react at 120°C for 2h, then raise the temperature to 150°C, and continue to react for 3h. When the acid value dropped to 104.4mgKOH / g resin, the reaction was stopped to obtain epoxy...
Embodiment 2
[0054] (1) Under nitrogen protection, add hydroxyethyl acrylate HEA or hydroxypropyl acrylate HPA and dibutyltin dilaurate (DBTDL) dropwise to toluene diisocyanate (TDI), keep the reaction temperature at 45°C, and monitor the system - The concentration of NCO changes until the -NCO concentration does not change anymore, the molar ratio of HEA or HPA to diisocyanate is 1: 1.05; the mass ratio of dibutyltin dilaurate to hydroxyethyl acrylate or hydroxypropyl acrylate is 1.0: 100, Obtain the semi-adduct of diisocyanate;
[0055] (2) The ring-opening esterification reaction of epoxy resin E44 and tricarboxybenzenesulfonic acid under the effect of catalyst triphenylphosphine, the mol ratio of epoxy resin and tricarboxybenzenesulfonic acid is 1: 2, and the initial acid value is 433.6mgKOH / g resin, reacted at 125°C for 1.5h, then raised the temperature to 150°C, and continued to react for 3.5h. When the acid value dropped to 325.2mgKOH / g resin, the reaction was stopped to obtain epo...
Embodiment 3
[0059] (1) Under the protection of nitrogen, add hydroxyethyl acrylate HEA or hydroxypropyl acrylate HPA and dibutyltin dilaurate (DBTDL) dropwise to hexamethylene diisocyanate (HDI), keep the reaction temperature at 50°C, monitor The concentration of -NCO in the system changes until the concentration of -NCO no longer changes, the molar ratio of HEA or HPA to diisocyanate is 1:1; the mass ratio of dibutyltin dilaurate to hydroxyethyl acrylate or hydroxypropyl acrylate is 1.1 : 100, to obtain the semi-adduct of diisocyanate;
[0060] (2) The ring-opening esterification reaction of epoxy resin E51 and tricarboxybenzenesulfonic acid under the effect of catalyst triphenylphosphine, the mol ratio of epoxy resin and tricarboxybenzenesulfonic acid is 3: 1, and the initial acid value is 135.68mgKOH / g resin, reacted at 120°C for 2h, then raised the temperature to 150°C, and continued to react for 3.5h. When the acid value dropped to 33.92mgKOH / g resin, the reaction was stopped to obt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com