Chemical solution preparation method for tungsten molybdate solid solution luminescent microcrystal
A technology of tungstomolybdate and chemical solution, which is applied in the field of scheelite structure luminescent materials, to achieve the effect of expanding the application field, low reaction temperature, and good application and promotion prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Ca(MoO 4 ) x (WO 4 ) (1-x) Preparation of Solid Solution Luminescent Microcrystals
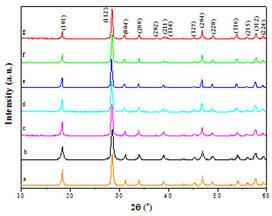
[0042] According to the preparation of a certain amount of target system Ca(MoO 4 ) x (WO 4 ) (1-x) The molar ratio of various raw materials required for microcrystals, and the corresponding volume of Ca(NO 3 ) 2 solution in a polytetrafluoroethylene beaker, and then add the corresponding amount of Na 2 MoO 4 and Na 2 WO 4 Solution, stirred at room temperature for 5-20 minutes, then put the polytetrafluoroethylene beaker into the autoclave. After hydrothermal reaction at 60-180° C. for 15-30 hours, naturally cool to room temperature. The precipitate is centrifuged, washed with deionized water, and then dried at 100-150°C to obtain Ca(MoO 4 ) x (WO 4 ) (1-x) Solid solution luminescent microcrystals. The X-ray diffraction pattern of the crystallite is as figure 1 As shown, all crystallites are tetragonal single-phase structure of scheelite, ind...
Embodiment 2
[0044] Example 2: Sr(MoO 4 ) x (WO 4 ) (1-x) Preparation of Solid Solution Luminescent Microcrystals
[0045] Divide Ca (NO 3 ) 2 replaced by Sr (NO 3 ) 2 Outside, all the other press embodiment 1 technique, prepare Sr(MoO 4 ) x (WO 4 ) (1-x) series of solid solution crystallites.
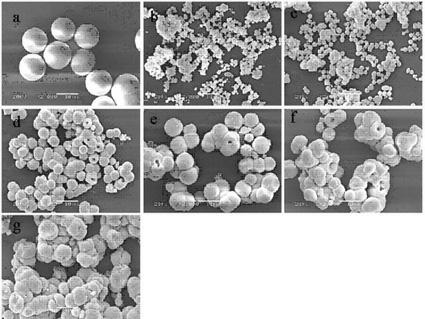
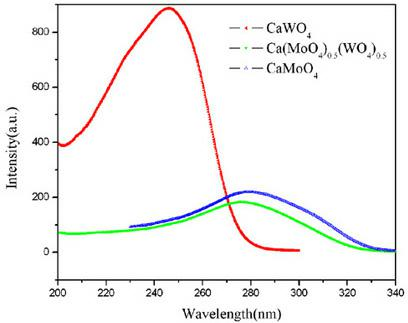
[0046] Figure 5 For the prepared Sr(MoO 4 ) x (WO 4 ) (1-x) X-ray diffraction pattern of the crystallite. showed that a pure scheelite-structured Sr(MoO 4 ) x (WO 4 ) (1-x) solid solution. Figure 6 The preparation of Sr(MoO 4 ) x (WO 4 ) (1-x) SEM topography of solid solution crystallites. It can be seen that the microcrystals are all spherical particles with a uniform particle size, and the average size is about 10 μm; and the surface of the microcrystal particles is smooth, which is conducive to coating a uniform, dense and smooth luminescent layer. Figure 7 gives Sr(MoO 4 ) x (WO 4 ) (1-x) Room temperature photoluminescence spectra (PL spectra) ...
Embodiment 3
[0047] Example 3: Ba(MoO 4 ) x (WO 4 ) (1-x) Preparation of Solid Solution Luminescent Microcrystals
[0048] In addition to Ca(NO 3 ) 2 replaced by Ba(NO 3 ) 2 Outside, all the other press embodiment 1 technique, prepare Ba(MoO 4 ) x (WO 4 ) (1-x) A series of solid solution luminescent microcrystals. The X-ray diffraction pattern of the crystallite is as Figure 8 As shown, it can be seen that the crystallites obtained are all tetragonal single-phase structures of scheelite, indicating that they formed Ba(MoO 4 ) x (WO 4 ) (1-x) solid solution. Figure 9 The preparation of Ba(MoO 4 ) x (WO 4 ) (1-x) SEM images of microcrystals. It can be seen that the morphology of the prepared microcrystals is square pyramid or biconical, and a small amount of agglomeration occurs, which can be improved by adding surfactants.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com